Abstract
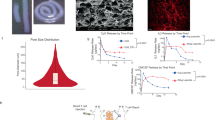
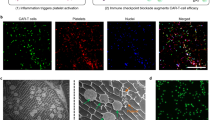
A tumour microenvironment abundant in regulatory T (Treg) cells aids solid tumours to evade clearance by effector T cells. Systemic strategies to suppress Treg cells or to augment immunity can elicit autoimmune side effects, cytokine storms and other toxicities. Here we report the design, fabrication and therapeutic performance of a biodegradable macroporous scaffold, implanted peritumourally, that releases a small-molecule inhibitor of transforming growth factor β to suppress Treg cells, chemokines to attract effector T cells and antibodies to stimulate them. In two mouse models of aggressive tumours, the implant boosted the recruitment and activation of effector T cells into the tumour and depleted it of Treg cells, which resulted in an ‘immunological abscopal effect’ on distant metastases and in the establishment of long-term memory that impeded tumour recurrence. We also show that the scaffold can be used to deliver tumour-antigen-specific T cells into the tumour. Peritumourally implanted immunomodulatory scaffolds may represent a general strategy to enhance T-cell immunity and avoid the toxicities of systemic therapies.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
$99.00 per year
only $8.25 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
Data availability
The main data supporting the results in this study are available within the paper and its Supplementary Information. All data generated in this study, including source data for the figures, are available from figshare with the identifier https://doi.org/10.6084/m9.figshare.21406713.
References
Dahan, R. et al. FcγRs modulate the anti-tumor activity of antibodies targeting the PD-1/PD-L1 axis. Cancer Cell 28, 285–295 (2015).
Simpson, T. R. et al. Fc-dependent depletion of tumor-infiltrating regulatory T cells co-defines the efficacy of anti-CTLA-4 therapy against melanoma. J. Exp. Med. 210, 1695–1710 (2013).
Haanen, J. B. A. G. et al. Management of toxicities from immunotherapy: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann. Oncol. 28, iv119–iv142 (2017).
Champiat, S. et al. Management of immune checkpoint blockade dysimmune toxicities: a collaborative position paper. Ann. Oncol. 27, 559–574 (2016).
Naidoo, J. et al. Toxicities of the anti-PD-1 and anti-PD-L1 immune checkpoint antibodies. Ann. Oncol. 27, 1362 (2016).
Kumagai, S. et al. The PD-1 expression balance between effector and regulatory T cells predicts the clinical efficacy of PD-1 blockade therapies. Nat. Immunol. 21, 1346–1358 (2020).
Sasada, T., Kimura, M., Yoshida, Y., Kanai, M. & Takabayashi, A. CD4+CD25+ regulatory T cells in patients with gastrointestinal malignancies: possible involvement of regulatory T cells in disease progression. Cancer 98, 1089–1099 (2003).
Curiel, T. J. et al. Specific recruitment of regulatory T cells in ovarian carcinoma fosters immune privilege and predicts reduced survival. Nat. Med. 10, 942–949 (2004).
Sato, E. et al. Intraepithelial CD8+ tumor-infiltrating lymphocytes and a high CD8+/regulatory T cell ratio are associated with favorable prognosis in ovarian cancer. Proc. Natl Acad. Sci. USA 102, 18538–18543 (2005).
Shang, B., Liu, Y., Jiang, S. J. & Liu, Y. Prognostic value of tumor-infiltrating Foxp3+ regulatory T cells in cancers: a systematic review and meta-analysis. Sci. Rep. 5, 15179 (2015).
Xydia, M. et al. Common clonal origin of conventional T cells and induced regulatory T cells in breast cancer patients. Nat. Commun. 12, 1119 (2021).
Thomas, D. A. & Massagué, J. TGF-β directly targets cytotoxic T cell functions during tumor evasion of immune surveillance. Cancer Cell 8, 369–380 (2005).
Mariathasan, S. et al. TGFβ attenuates tumour response to PD-L1 blockade by contributing to exclusion of T cells. Nature 554, 544–548 (2018).
Batlle, E. & Massagué, J. Transforming growth factor-β signaling in immunity and cancer. Immunity 50, 924–940 (2019).
Stauber, A. J., Credille, K. M., Truex, L. L., Ehlhardt, W. J. & Young, J. K. Nonclinical safety evaluation of a transforming growth factor β receptor I kinase inhibitor in Fischer 344 rats and beagle dogs. J. Clin. Toxicol. 4, 1–10 (2014).
Herbertz, S. et al. Clinical development of galunisertib (Ly2157299 monohydrate), a small molecule inhibitor of transforming growth factor-beta signaling pathway. Drug Des. Devel. Ther. 9, 4479–4499 (2015).
Park, J. et al. Combination delivery of TGF-β inhibitor and IL-2 by nanoscale liposomal polymeric gels enhances tumour immunotherapy. Nat. Mater. 11, 895–905 (2012).
Colombo, M. P. & Piconese, S. Regulatory T-cell inhibition versus depletion: the right choice in cancer immunotherapy. Nat. Rev. Cancer 7, 880–887 (2007).
Zhou, X. et al. Precise spatiotemporal interruption of regulatory T-cell-mediated CD8+ T-cell suppression leads to tumor immunity. Cancer Res. 79, 585–597 (2019).
Onizuka, S. et al. Tumor rejection by in vivo administration of anti-CD25 (interleukin-2 receptor α) monoclonal antibody. Cancer Res. 59, 3128–3133 (1999).
Tanaka, A. & Sakaguchi, S. Targeting Treg cells in cancer immunotherapy. Eur. J. Immunol. 49, 1140–1146 (2019).
Sato, K. et al. Spatially selective depletion of tumor-associated regulatory T cells with near-infrared photoimmunotherapy. Sci. Transl. Med. 8, 352ra110 (2016).
Meng, K. P., Majedi, F. S., Thauland, T. J. & Butte, M. J. Mechanosensing through YAP controls T cell activation and metabolism. J. Exp. Med. 217, e20200053 (2020).
Majedi, F. S. et al. T-cell activation is modulated by the 3D mechanical microenvironment. Biomaterials 252, 120058 (2020).
Khazaie, K. & von Boehmer, H. The impact of CD4+CD25+ Treg on tumor specific CD8+ T cell cytotoxicity and cancer. Semin. Cancer Biol. 16, 124–136 (2006).
Budhu, S. et al. Blockade of surface-bound TGF-β on regulatory T cells abrogates suppression of effector T cell function in the tumor microenvironment. Sci. Signal. 10, eaak9702 (2017).
Pulaski, B. A. & Ostrand-Rosenberg, S. Mouse 4T1 breast tumor model. Curr. Protoc. Immunol. https://doi.org/10.1002/0471142735.im2002s39 (2001).
Han, J., Khatwani, N., Searles, T. G., Turk, M. J. & Angeles, C. V. Memory CD8+ T cell responses to cancer. Semin. Immunol. 49, 101435 (2020).
Wrzesinski, S. H., Wan, Y. Y. & Flavell, R. A. Transforming growth factor-β and the immune response: implications for anticancer therapy. Clin. Cancer Res. 13, 5262–5270 (2007).
Novak, L., Igoucheva, O., Cho, S. & Alexeev, V. Characterization of the CCL21-mediated melanoma-specific immune responses and in situ melanoma eradication. Mol. Cancer Ther. 6, 1755–1764 (2007).
Riley, R. S., June, C. H., Langer, R. & Mitchell, M. J. Delivery technologies for cancer immunotherapy. Nat. Rev. Drug Discov. 18, 175–196 (2019).
Smith, T. T. et al. Biopolymers codelivering engineered T cells and STING agonists can eliminate heterogeneous tumors. J. Clin. Invest. 127, 2176–2191 (2017).
Stephan, S. B. et al. Biopolymer implants enhance the efficacy of adoptive T-cell therapy. Nat. Biotechnol. 33, 97–101 (2015).
Tahmasebi, S., Elahi, R. & Esmaeilzadeh, A. Solid tumors challenges and new insights of CAR T cell engineering. Stem Cell Rev. Rep. 15, 619–636 (2019).
Tang, L. et al. Enhancing T cell therapy through TCR-signaling-responsive nanoparticle drug delivery. Nat. Biotechnol. https://doi.org/10.1038/nbt.4181 (2018).
Mansurov, A. et al. Collagen-binding IL-12 enhances tumour inflammation and drives the complete remission of established immunologically cold mouse tumours. Nat. Biomed. Eng. https://doi.org/10.1038/s41551-020-0549-2 (2020).
Majedi, F. S. et al. Cytokine secreting microparticles engineer the fate and the effector functions of T-cells. Adv. Mater. 30, 1703178 (2018).
Stohrer, M., Boucher, Y., Stangassinger, M. & Jain, R. K. Oncotic pressure in solid tumors is elevated. Cancer Res. 60, 4251–4255 (2000).
Jain, R. K. Physiological barriers to delivery of monoclonal antibodies and other macromolecules in tumors. Cancer Res. 50, 814–819 (1990).
Adusumilli, P. S. et al. Regional delivery of mesothelin-targeted CAR T cell therapy generates potent and long-lasting CD4-dependent tumor immunity. Sci. Transl. Med. 6, 261ra151 (2014).
Katz, S. C. et al. Regional CAR-T cell infusions for peritoneal carcinomatosis are superior to systemic delivery. Cancer Gene Ther. 23, 142–148 (2016).
Nellan, A. et al. Durable regression of medulloblastoma after regional and intravenous delivery of anti-HER2 chimeric antigen receptor T cells. J. Immunother. Cancer 6, 1–14 (2018).
Waldron, K. et al. Formation of monodisperse mesoporous silica microparticles via spray-drying. J. Colloid Interface Sci. https://doi.org/10.1016/j.jcis.2013.12.027 (2014).
Liu, W., Chen, X. D. & Selomulya, C. On the spray drying of uniform functional microparticles. Particuology 22, 1–12 (2015).
Dearman, R. J., Caddick, H., Basketter, D. A. & Kimber, I. Divergent antibody isotype responses induced in mice by systemic exposure to proteins: a comparison of ovalbumin with bovine serum albumin. Food Chem. Toxicol. 38, 351–360 (2000).
Hasani-Sadrabadi, M. M. et al. On-chip synthesis of fine-tuned bone-seeking hybrid nanoparticles. Nanomedicine 10, 3431–3449 (2015).
Sterilization of Health Care Products—Radiation—Part 2: Establishing the Sterilization Dose—Technical Corrigendum 1 (ISO, 2009).
Bellone, M. et al. Relevance of the tumor antigen in the validation of three vaccination strategies for melanoma. J. Immunol. 165, 2651–2656 (2000).
Acknowledgements
We thank L. Bentolila and S. Xu for their technical assistance with confocal imaging and animal imaging studies, respectively, and S. Haile for her assistance with flow cytometry. M.J.B. discloses support in part for the research described in this study from the University of California, Los Angeles (UCLA) Jonsson Comprehensive Cancer Center (JCCC). L.-S.B. discloses support in part for the research described in this study from the UCLA CTSI (UL1TR001881). S.L. discloses support in part for the research described in this study from the National Institutes of Health (NIH) (R01 CA234343). F.S.M. discloses support in part for the research described in this study from the R&D fund by Symphony Bioscience. Confocal laser scanning microscopy was performed at the Advanced Light Microscopy/Spectroscopy Laboratory and the Leica Microsystems Centre of Excellence at the California NanoSystems Institute at UCLA, which is supported by funding from the NIH Shared Instrumentation Grant (S10OD025017) and NSF Major Research Instrumentation grant (CHE-0722519). Scanning electron microscopy was performed at California NanoSystems Institute Electron Imaging Centre for NanoMachines Shared Resource Facility at UCLA. Flow cytometry was partially performed at the UCLA JCCC and Centre for AIDS Research Flow Cytometry Core Facility, which are partially supported by NIH (P30 CA016042 and 5P30 AI028697), and by the JCCC, the UCLA AIDS Institute, the David Geffen School of Medicine at UCLA, the UCLA Chancellor’s Office and the UCLA Vice Chancellor’s Office of Research. The contents of this publication are solely the responsibility of the authors and do not necessarily represent the official views of NIH (United States) or/and other agencies of the State of California.
Author information
Authors and Affiliations
Contributions
F.S.M., M.M.H.-S., L.-S.B., S.L. and M.J.B. conceived and designed the experiments. F.S.M, M.M.H.-S. and T.J.T. performed the experiments. S.G.K. performed terminal deoxynucleotidyl transferase-mediated dUTP nick-end labelling staining. F.S.M, M.M.H.-S. and M.J.B. analysed the data and wrote the manuscript. All authors discussed the results and commented on the manuscript. M.J.B. supervised all work.
Corresponding authors
Ethics declarations
Competing interests
The Regents of the University of California filed patent applications (PCT/US2020/051363 and WO2021055658A1) related to this study, with F.S.M, M.M.H.-S. and M.J.B. as inventors. F.S.M, M.M.H.-S. and M.J.B. are founders and equity holders in Symphony Biosciences. The other authors declare no competing interests.
Peer review
Peer review information
Nature Biomedical Engineering thanks the anonymous reviewers for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
Supplementary figures and tables.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Majedi, F.S., Hasani-Sadrabadi, M.M., Thauland, T.J. et al. Systemic enhancement of antitumour immunity by peritumourally implanted immunomodulatory macroporous scaffolds. Nat. Biomed. Eng 7, 56–71 (2023). https://doi.org/10.1038/s41551-022-00977-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41551-022-00977-0
This article is cited by
-
Biomaterials to enhance adoptive cell therapy
Nature Reviews Bioengineering (2024)