Abstract
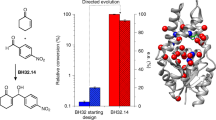
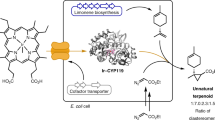
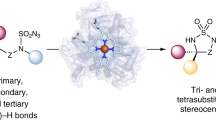
Recent advances in enzyme engineering and design have expanded nature’s catalytic repertoire to functions that are new to biology1,2,3. However, only a subset of these engineered enzymes can function in living systems4,5,6,7. Finding enzymatic pathways that form chemical bonds that are not found in biology is particularly difficult in the cellular environment, as this depends on the discovery not only of new enzyme activities, but also of reagents that are both sufficiently reactive for the desired transformation and stable in vivo. Here we report the discovery, evolution and generalization of a fully genetically encoded platform for producing chiral organoboranes in bacteria. Escherichia coli cells harbouring wild-type cytochrome c from Rhodothermus marinus8 (Rma cyt c) were found to form carbon–boron bonds in the presence of borane–Lewis base complexes, through carbene insertion into boron–hydrogen bonds. Directed evolution of Rma cyt c in the bacterial catalyst provided access to 16 novel chiral organoboranes. The catalyst is suitable for gram-scale biosynthesis, providing up to 15,300 turnovers, a turnover frequency of 6,100 h–1, a 99:1 enantiomeric ratio and 100% chemoselectivity. The enantiopreference of the biocatalyst could also be tuned to provide either enantiomer of the organoborane products. Evolved in the context of whole-cell catalysts, the proteins were more active in the whole-cell system than in purified forms. This study establishes a DNA-encoded and readily engineered bacterial platform for borylation; engineering can be accomplished at a pace that rivals the development of chemical synthetic methods, with the ability to achieve turnovers that are two orders of magnitude (over 400-fold) greater than those of known chiral catalysts for the same class of transformation9,10,11. This tunable method for manipulating boron in cells could expand the scope of boron chemistry in living systems.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Renata, H., Wang, Z. J. & Arnold, F. H. Expanding the enzyme universe: accessing non-natural reactions by mechanism-guided directed evolution. Angew. Chem. Int. Ed. 54, 3351–3367 (2015)
Hyster, T. K. & Ward, T. R. Genetic optimization of metalloenzymes: enhancing enzymes for non-natural reactions. Angew. Chem. Int. Ed. 55, 7344–7357 (2016)
Hammer, S. C., Knight, A. M. & Arnold, F. H. Design and evolution of enzymes for non-natural chemistry. Curr. Opin. Green Sustainable Chem. 7, 23–30 (2017)
Coelho, P. S. et al. A serine-substituted P450 catalyzes highly efficient carbene transfer to olefins in vivo. Nat. Chem. Biol. 9, 485–487 (2013)
Jeschek, M. et al. Directed evolution of artificial metalloenzymes for in vivo metathesis. Nature 537, 661–665 (2016)
Kan, S. B. J., Lewis, R. D., Chen, K. & Arnold, F. H. Directed evolution of cytochrome c for carbon–silicon bond formation: Bringing silicon to life. Science 354, 1048–1051 (2016)
Tinoco, A., Steck, V., Tyagi, V. & Fasan, R. Highly diastereo- and enantioselective synthesis of trifluoromethyl-substituted cyclopropanes via myoglobin-catalyzed transfer of trifluoromethylcarbene. J. Am. Chem. Soc. 139, 5293–5296 (2017)
Stelter, M. et al. A novel type of monoheme cytochrome c: biochemical and structural characterization at 1.23 A resolution of Rhodothermus marinus cytochrome c. Biochemistry 47, 11953–11963 (2008)
Cheng, Q.-Q., Zhu, S.-F., Zhang, Y.-Z., Xie, X.-L. & Zhou, Q.-L. Copper-catalyzed B–H bond insertion reaction: a highly efficient and enantioselective C–B bond-forming reaction with amine–borane and phosphine–borane adducts. J. Am. Chem. Soc. 135, 14094–14097 (2013)
Chen, D., Zhang, X., Qi, W.-Y., Xu, B. & Xu, M.-H. Rhodium(i)-catalyzed asymmetric carbene insertion into B–H bonds: highly enantioselective access to functionalized organoboranes. J. Am. Chem. Soc. 137, 5268–5271 (2015)
Hyde, S. et al. Copper-catalyzed insertion into heteroatom–hydrogen bonds with trifluorodiazoalkanes. Angew. Chem. Int. Ed. 55, 3785–3789 (2016)
Irschik, H., Schummer, D., Gerth, K., Höfle, G. & Reichenbach, H. The tartrolons, new boron-containing antibiotics from a myxobacterium, Sorangium cellulosum J. Antibiot. 48, 26–30 (1995)
Wolkenstein, K., Sun, H., Falk, H. & Griesinger, C. Structure and absolute configuration of Jurassic polyketide-derived spiroborate pigments obtained from microgram quantities. J. Am. Chem. Soc. 137, 13460–13463 (2015)
Chen, X. et al. Structural identification of a bacterial quorum-sensing signal containing boron. Nature 415, 545–549 (2002)
Elshahawi, S. I. et al. Boronated tartrolon antibiotic produced by symbiotic cellulose-degrading bacteria in shipworm gills. Proc. Natl Acad. Sci. USA 110, E295–E304 (2013)
Dembitsky, V. M., Al Quntar, A. A. & Srebnik, M. Natural and synthetic small boron-containing molecules as potential inhibitors of bacterial and fungal quorum sensing. Chem. Rev. 111, 209–237 (2011)
Prier, C. K., Zhang, R. K., Buller, A. R., Brinkmann-Chen, S. & Arnold, F. H. Enantioselective, intermolecular benzylic C–H amination catalysed by an engineered iron-haem enzyme. Nat. Chem. 9, 629–634 (2017)
Das, B. C. et al. Boron chemicals in diagnosis and therapeutics. Future Med. Chem. 5, 653–676 (2013)
Miyaura, N. & Suzuki, A. Palladium-catalyzed cross-coupling reactions of organoboron compounds. Chem. Rev. 95, 2457–2483 (1995)
Leonori, D. & Aggarwal, V. K. Lithiation–borylation methodology and its application in synthesis. Acc. Chem. Res. 47, 3174–3183 (2014)
Leonori, D. & Aggarwal, V. K. Stereospecific couplings of secondary and tertiary boronic esters. Angew. Chem. Int. Ed. 54, 1082–1096 (2015)
Tehfe, M. A. et al. A water-compatible NHC-borane: Photopolymerizations in water and rate constants for elementary radical reactions. ACS Macro Lett. 1, 92–95 (2012)
Handa, S., Wang, Y., Gallou, F. & Lipshutz, B. H. Sustainable Fe–ppm Pd nanoparticle catalysis of Suzuki-Miyaura cross-couplings in water. Science 349, 1087–1091 (2015)
Chang, M. C. Y., Pralle, A., Isacoff, E. Y. & Chang, C. J. A selective, cell-permeable optical probe for hydrogen peroxide in living cells. J. Am. Chem. Soc. 126, 15392–15393 (2004)
Halo, T. L., Appelbaum, J., Hobert, E. M., Balkin, D. M. & Schepartz, A. Selective recognition of protein tetraserine motifs with a cell-permeable, pro-fluorescent bis-boronic acid. J. Am. Chem. Soc. 131, 438–439 (2009)
Kim, J. & Bertozzi, C. R. A bioorthogonal reaction of N-oxide and boron reagents. Angew. Chem. Int. Ed. 54, 15777–15781 (2015)
Li, X. & Curran, D. P. Insertion of reactive rhodium carbenes into boron–hydrogen bonds of stable N-heterocyclic carbene boranes. J. Am. Chem. Soc. 135, 12076–12081 (2013)
Curran, D. P. et al. Synthesis and reactions of N-heterocyclic carbene boranes. Angew. Chem. Int. Ed. 50, 10294–10317 (2011)
Würtemberger-Pietsch, S., Radius, U. & Marder, T. B. 25 years of N-heterocyclic carbenes: activation of both main-group element–element bonds and NHCs themselves. Dalton Trans. 45, 5880–5895 (2016)
Arslan, E ., Schulz, H ., Zufferey, R ., Künzler, P & Thöny-Meyer, L. Overproduction of the Bradyrhizobium japonicum c-type cytochrome subunits of the cbb3 oxidase in Escherichia coli. Biochem. Biophys. Res. Commun. 251, 744–747 (1998)
Kille, S. et al. Reducing codon redundancy and screening effort of combinatorial protein libraries created by saturation mutagenesis. ACS Synth. Biol. 2, 83–92 (2013)
Mara, M. W. et al. Metalloprotein entatic control of ligand–metal bonds quantified by ultrafast X-ray spectroscopy. Science 356, 1276–1280 (2017)
Renata, H. et al. Identification of mechanism-based inactivation in P450-catalyzed cyclopropanation facilitates engineering of improved enzymes. J. Am. Chem. Soc. 138, 12527–12533 (2016)
Hernandez, K. E. et al. Highly stereoselective biocatalytic synthesis of key cyclopropane intermediate to ticagrelor. ACS Catal. 6, 7810–7813 (2016)
Argintaru, O. A., Ryu, D., Aron, I. & Molander, G. A. Synthesis and applications of α-trifluoromethylated alkylboron compounds. Angew. Chem. Int. Ed. 52, 13656–13660 (2013)
Jiang, Q., Guo, T. & Yu, Z. Copper-catalyzed asymmetric borylation: Construction of a stereogenic carbon center bearing both CF3 and organoboron functional groups. J. Org. Chem. 82, 1951–1960 (2017)
Kanouni, T., Stafford, J. A., Veal, J. M. & Wallace, M. B. Histone demethylase inhibitors. WO 2014/151106 A1 (2014)
Scopes, D. Pyrrolo [3,2-E] [1,2,4] triazolo [1,5-A] pyrimidines derivatives as inhibitors of microglia activation. US patent 2012/0289523 A1 (2012)
Gibson, D. G. et al. Enzymatic assembly of DNA molecules up to several hundred kilobases. Nat. Methods 6, 343–345 (2009)
Sambrook, J ., Frisch, E. & Maniatis, T. Molecular Cloning: A Laboratory Manual (Cold Spring Harbor Laboratory Press, 1989)
Berry, E. A. & Trumpower, B. L. Simultaneous determination of hemes a, b, and c from pyridine hemochrome spectra. Anal. Biochem. 161, 1–15 (1987)
Yang, J.-M. et al. Catalytic B−H bond insertion reactions using alkynes as carbene precursors. J. Am. Chem. Soc. 139, 3784–3789 (2017)
Allen, T. H., Kawamoto, T., Gardner, S., Geib, S. J. & Curran, D. P. N-heterocyclic carbene boryl iodides catalyze insertion reactions of N-heterocyclic carbene boranes and diazoesters. Org. Lett. 19, 3680–3683 (2017)
Wang, Z. J. et al. Improved cyclopropanation activity of histidine-ligated cytochrome P450 enables the enantioselective formal synthesis of levomilnacipran. Angew. Chem. Int. Ed. 53, 6810–6813 (2014)
Acknowledgements
This work was supported in part by the National Science Foundation, Office of Chemical, Bioengineering, Environmental and Transport Systems SusChEM Initiative (grant CBET-1403077), the Gordon and Betty Moore Foundation through grant GBMF2809 to the Caltech Programmable Molecular Technology Initiative, and the Jacobs Institute for Molecular Engineering for Medicine at Caltech. X.H. is supported by a Ruth L. Kirschstein National Institutes of Health Postdoctoral Fellowship (F32GM125231). We thank O. F. Brandenberg, S. Brinkmann-Chen, T. Hashimoto, R. D. Lewis, and D. K. Romney for discussions and/or comments on the manuscript, and N. W. Goldberg and A. Zutshi for experimental assistance. We are grateful to S. Virgil, N. Torian, M. K. Takase and L. Henling for analytical support, and H. Gray for providing the pEC86 plasmid.
Author information
Authors and Affiliations
Contributions
S.B.J.K. and X.H. designed the research with guidance from F.H.A. S.B.J.K., X.H., Y.G. and K.C. performed the experiments and analysed the data. S.B.J.K., X.H. and F.H.A. wrote the manuscript with input from all authors.
Corresponding author
Ethics declarations
Competing interests
A provisional patent application has been filed through the California Institute of Technology based on the results presented here.
Additional information
Reviewer Information Nature thanks M. Fischbach and the other anonymous reviewer(s) for their contribution to the peer review of this work.
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data figures and tables
Extended Data Figure 2 Summary of known catalytic systems for metal–carbenoid insertion reactions of boranes.
a, Rh2(esp)2-catalysed borylation of diazo esters with NHC-boranes27. b, Cu(MeCN)4PF6-catalysed borylation of diazo esters with phosphine-borane9. c, [Rh(C2H4)2Cl]2-catalysed borylation of diazo esters with amine-borane10. d, Cu(MeCN)4PF6-catalysed borylation of CF3-substituted (diazomethyl)benzene with phosphine-borane11. e, Rh2(R-BTPCP)4-catalysed borylation using alkynes as carbene precursors42. f, I2-catalysed borylation of diazo esters with NHC-boranes43.
Extended Data Figure 3 Effect of biological borylation on E. coli cell viability.
Cell viability assay was performed in biological triplicate, see Methods section for experimental protocol.
Supplementary information
Supplementary Information
This file contains information on borylation with additional figures. (PDF 10741 kb)
Supplementary Data
This file contains a checkcif file for CCDC1572198. (PDF 282 kb)
Supplementary Data
This file contains a checkcif file for CCDC1572200. (PDF 302 kb)
Supplementary Data
This file contains a checkcif file for CCDC1572201. (PDF 306 kb)
Supplementary Data
This file contains cif files for structures CCDC1572198, CCDC1572200 and CCDC1572201. (ZIP 457 kb)
Rights and permissions
About this article
Cite this article
Kan, S., Huang, X., Gumulya, Y. et al. Genetically programmed chiral organoborane synthesis. Nature 552, 132–136 (2017). https://doi.org/10.1038/nature24996
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nature24996
This article is cited by
-
Reaction engineering blocks ether cleavage for synthesizing chiral cyclic hemiacetals catalyzed by unspecific peroxygenase
Nature Communications (2024)
-
Structures of the CcmABCD heme release complex at multiple states
Nature Communications (2022)
-
Engineering new catalytic activities in enzymes
Nature Catalysis (2020)
-
Emerging Frontiers in the Study of Molecular Evolution
Journal of Molecular Evolution (2020)
-
In vivo continuous evolution of metabolic pathways for chemical production
Microbial Cell Factories (2019)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.