Abstract
Long-term potentiation (LTP) of excitatory synaptic transmission has long been considered a cellular correlate for learning and memory1,2. Early LTP (less than 1 h) had initially been explained either by presynaptic increases in glutamate release3,4,5 or by direct modification of postsynaptic AMPA (α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid) receptor function6,7. Compelling models have more recently proposed that synaptic potentiation can occur by the recruitment of additional postsynaptic AMPA receptors (AMPARs)8, sourced either from an intracellular reserve pool by exocytosis or from nearby extra-synaptic receptors pre-existing on the neuronal surface9,10,11,12. However, the exact mechanism through which synapses can rapidly recruit new AMPARs during early LTP remains unknown. In particular, direct evidence for a pivotal role of AMPAR surface diffusion as a trafficking mechanism in synaptic plasticity is still lacking. Here, using AMPAR immobilization approaches, we show that interfering with AMPAR surface diffusion markedly impairs synaptic potentiation of Schaffer collaterals and commissural inputs to the CA1 area of the mouse hippocampus in cultured slices, acute slices and in vivo. Our data also identify distinct contributions of various AMPAR trafficking routes to the temporal profile of synaptic potentiation. In addition, AMPAR immobilization in vivo in the dorsal hippocampus inhibited fear conditioning, indicating that AMPAR diffusion is important for the early phase of contextual learning. Therefore, our results provide a direct demonstration that the recruitment of new receptors to synapses by surface diffusion is a critical mechanism for the expression of LTP and hippocampal learning. Since AMPAR surface diffusion is dictated by weak Brownian forces that are readily perturbed by protein–protein interactions, we anticipate that this fundamental trafficking mechanism will be a key target for modulating synaptic potentiation and learning.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Takeuchi, T., Duszkiewicz, A. J. & Morris, R. G. The synaptic plasticity and memory hypothesis: encoding, storage and persistence. Phil. Trans. R. Soc. Lond. B 369, 20130288 (2013)
Nicoll, R. A. A brief history of long-term potentiation. Neuron 93, 281–290 (2017)
MacDougall, M. J. & Fine, A. The expression of long-term potentiation: reconciling the preists and the postivists. Phil. Trans. R. Soc. Lond. B 369, 2013 0135 (2013)
Padamsey, Z. & Emptage, N. Two sides to long-term potentiation: a view towards reconciliation. Phil. Trans. R. Soc. Lond. B 369, 20130154 (2013)
Yang, Y. & Calakos, N. Presynaptic long-term plasticity. Front. Synaptic Neurosci. 5, 8 (2013)
Lisman, J., Yasuda, R. & Raghavachari, S. Mechanisms of CaMKII action in long-term potentiation. Nat. Rev. Neurosci. 13, 169–182 (2012)
Lu, W. & Roche, K. W. Posttranslational regulation of AMPA receptor trafficking and function. Curr. Opin. Neurobiol. 22, 470–479 (2012)
Granger, A. J. & Nicoll, R. A. Expression mechanisms underlying long-term potentiation: a postsynaptic view, 10 years on. Phil. Trans. R. Soc. Lond. B 369, 20130136 (2013)
Huganir, R. L. & Nicoll, R. A. AMPARs and synaptic plasticity: the last 25 years. Neuron 80, 704–717 (2013)
Opazo, P. et al. CaMKII triggers the diffusional trapping of surface AMPARs through phosphorylation of stargazin. Neuron 67, 239–252 (2010)
Chater, T. E. & Goda, Y. The role of AMPA receptors in postsynaptic mechanisms of synaptic plasticity. Front. Cell. Neurosci. 8, 401 (2014)
Opazo, P. & Choquet, D. A three-step model for the synaptic recruitment of AMPA receptors. Mol. Cell. Neurosci. 46, 1–8 (2011)
Bliss, T. V. & Collingridge, G. L. Expression of NMDA receptor-dependent LTP in the hippocampus: bridging the divide. Mol. Brain 6, 5 (2013)
Lledo, P. M., Zhang, X., Südhof, T. C., Malenka, R. C. & Nicoll, R. A. Postsynaptic membrane fusion and long-term potentiation. Science 279, 399–403 (1998)
Park, M., Penick, E. C., Edwards, J. G., Kauer, J. A. & Ehlers, M. D. Recycling endosomes supply AMPA receptors for LTP. Science 305, 1972–1975 (2004)
Patterson, M. A., Szatmari, E. M. & Yasuda, R. AMPA receptors are exocytosed in stimulated spines and adjacent dendrites in a Ras-ERK-dependent manner during long-term potentiation. Proc. Natl Acad. Sci. USA 107, 15951–15956 (2010)
Wu, D. et al. Postsynaptic synaptotagmins mediate AMPA receptor exocytosis during LTP. Nature 544, 316–321 (2017)
Borgdorff, A. J. & Choquet, D. Regulation of AMPA receptor lateral movements. Nature 417, 649–653 (2002)
Granger, A. J., Shi, Y., Lu, W., Cerpas, M. & Nicoll, R. A. LTP requires a reserve pool of glutamate receptors independent of subunit type. Nature 493, 495–500 (2013)
Makino, H. & Malinow, R. AMPA receptor incorporation into synapses during LTP: the role of lateral movement and exocytosis. Neuron 64, 381–390 (2009)
Howarth, M., Takao, K., Hayashi, Y. & Ting, A. Y. Targeting quantum dots to surface proteins in living cells with biotin ligase. Proc. Natl Acad. Sci. USA 102, 7583–7588 (2005)
Williams, K. Modulation and block of ion channels: a new biology of polyamines. Cell. Signal. 9, 1–13 (1997)
Liu, X. et al. Optogenetic stimulation of a hippocampal engram activates fear memory recall. Nature 484, 381–385 (2012)
McHugh, T. J. et al. Dentate gyrus NMDA receptors mediate rapid pattern separation in the hippocampal network. Science 317, 94–99 (2007)
Takemoto, K. et al. Optical inactivation of synaptic AMPA receptors erases fear memory. Nat. Biotechnol. 35, 38–47 (2017)
Kessels, H. W. & Malinow, R. Synaptic AMPA receptor plasticity and behavior. Neuron 61, 340–350 (2009)
Whitlock, J. R., Heynen, A. J., Shuler, M. G. & Bear, M. F. Learning induces long-term potentiation in the hippocampus. Science 313, 1093–1097 (2006)
Giannone, G. et al. Dynamic superresolution imaging of endogenous proteins on living cells at ultra-high density. Biophys. J. 99, 1303–1310 (2010)
Jia, Z. et al. Enhanced LTP in mice deficient in the AMPA receptor GluR2. Neuron 17, 945–956 (1996)
Phair, R. D., Gorski, S. A. & Misteli, T. Measurement of dynamic protein binding to chromatin in vivo, using photobleaching microscopy. Methods Enzymol. 375, 393–414 (2004)
Penn, A. C., Balik, A., Wozny, C., Cais, O. & Greger, I. H. Activity-mediated AMPA receptor remodeling, driven by alternative splicing in the ligand-binding domain. Neuron 76, 503–510 (2012)
Rathenberg, J., Nevian, T. & Witzemann, V. High-efficiency transfection of individual neurons using modified electrophysiology techniques. J. Neurosci. Methods 126, 91–98 (2003)
Potier, M. et al. Temporary memory and its enhancement by estradiol requires surface dynamics of hippocampal CA1 N-methyl-D-aspartate receptors. Biol. Psychiatry 79, 734–745 (2016)
Acknowledgements
We would like to thank A. Ting (MIT) for providing the BirA-ER cDNA; E. Normand for histology; H. el Oussini for helping with acute slice experiments; A. Lacquemant, A. Gautier, M. Deshors and others at the Pôle In vivo in the IINS for animal husbandry; the Plateforme Génotypage in Neurocentre Magendie; M. Carta for insightful discussions; R. Sprengel for providing the Gria2-knockout mice; E. Gouaux for providing the anti-GluA2 antibodies, A. Carbone for data she obtained on a short-term EMBO fellowship. The help of the Bordeaux Imaging Center, part of the national infrastructure France BioImaging, granted by ANR-10INBS-04-0, is acknowledged. This work was funded by: EMBO long-term fellowship ALTF 129-2009 (A.C.P.); European Commission Marie Curie Actions FP7-PEOPLE-2010-IEF-273567 (A.C.P.), Medical Research Council Career Development Award fellowship MR/M020746/1 (A.C.P.), funding from the Ministère de l’Enseignement Supérieur et de la Recherche, Centre National de la Recherche Scientifique, the Conseil Régional d'Aquitaine, the Agence Nationale pour la Recherche Grant Nanodom and the ERC grants nano-dyn-syn and ADOS to D.C.
Author information
Authors and Affiliations
Contributions
A.C.P. performed molecular biology, imaging, slice culture and transfection, designed and executed slice electrophysiology experiments (all pertaining to the avidin–biotin crosslinking experiments) and wrote the paper. Y.H. and C.L.Z. performed acute slice electrophysiology using the antibody-mediated crosslinking approach. C.L.Z. performed surgeries and behavioural experiments. F.G. performed in vivo electrophysiology experiments. C.B. performed molecular and cell biology. J.D.P. performed antibody feeding experiments. E.H. performed single-particle tracking and Q-dot experiments. L.R. performed some imaging experiments. A.C.P. and Y.H. analysed the data. D.C. and Y.H. conceived and supervised the study. All authors discussed the results and contributed to the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Additional information
Reviewer Information Nature thanks R. C. Malenka and the other anonymous reviewer(s) for their contribution to the peer review of this work.
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data figures and tables
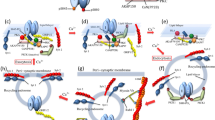
Extended Data Figure 1 Crosslink of AMPA receptors in cultured hippocampal neurons measured by quantum dot tracking.
NeutrAvidin and anti-GluA2 IgG (clone 14B11) crosslinking, to a similar extent, reduce the surface diffusion of bAP–SEP–GluA2 expressed in rat hippocampal neurons as measured by quantum dot tracking. Relative frequency histogram (left) of log-transformed diffusion coefficients (D), bar graph of mobile fraction (middle) and a plot of group data for mean-squared displacement (MSD) curves of all trajectories (right). Control was no antibody/NeutrAvidin. Bar graph shows mean ± s.e.m. Statistical significance was assessed by one-way ANOVA with Holm–Bonferroni post hoc tests. **P < 0.01; *** P < 0.001.
Extended Data Figure 2 Controls relating to crosslink by pre-treating bAP::SEP::GluA2-transfected slices cultures with NeutrAvidin.
a, Biotin-binding proteins diffuse through living organotypic slices and bind specifically to bAP–SEP–GluA2-expressing cells. Images show a maximum projection of an example 6.6 μm z-stack in a transfected Gria2−/− organotypic slice. CA1 neurons were co-transfected with tdTomato and bAP::SEP::GluA2. Streptavidin AF-633 staining was observed in all 12 bAP::SEP::GluA2-transfected CA1/3 cells observed and imaged. By contrast, no surface staining was observed in five CA1/3 cells transfected with myc::SEP::GluA2. b, c, Whole-cell recordings of bAP–SEP–GluA2-replacement CA1 neurons reveal stable baseline synaptic transmission (without high-frequency stimulation, that is, pseudo-HFS) in slices either with (c) or without (b) NeutrAvidin pre-treatment. Summary plots of mean normalized EPSP slope ± s.e.m. (left) and cumulative histograms of STP and LTP (right). d, Top, superimposed postsynaptic response of each cell during the first HFS train (grey) for control and NeutrAvidin pre-treatment groups; the average responses (after spikes were removed using a median filter with window width ranging from 2–6 ms) are shown in black and blue respectively. Bottom, bar graph showing no significant effect of NeutrAvidin pre-treatment on the area under curve (AUC) of the postsynaptic depolarization recorded across the three HFS trains used to induce synaptic potentiation. e, Bar graph showing no significant effect of NeutrAvidin pre-treatment on the EPSP slope during the baseline recording. f, No significant effect of NeutrAvidin pre-treatment on the amplitude and time course of NMDAR currents is detected in bAP–SEP–GluA2-replacement cells. Top, evoked NMDAR EPSCs were simultaneously recorded in bAP::SEP::GluA2-transfected and neighbouring untransfected cells of Gria2−/− slices. Example NMDAR-mediated EPSC recordings from transfected cells in control (left) and NeutrAvidin pre-treatment (right) groups. Two-exponent fits to the decay (dashed red lines) and the weighted average (bold red line) are superimposed over the traces. Bottom, scatter plots of NMDAR EPSC amplitude (above) and decay time constant (below) for transfected and untransfected cells from control (left) and NeutrAvidin pre-treatment (right) groups. Bold line represents the line of unity. Dashed lines represent 95% confidence bands from linear fits through the origin. The line of unity is between the confidence bounds in all cases. Bar graphs show means ± s.e.m. (d, e). Statistical significance was assessed by mixed model nested ANOVA (d) or two-way ANOVA without interaction (e). ns, not significant; *P < 0.05.
Extended Data Figure 3 Controls relating to manipulations preventing AMPAR diffusion and exocytosis.
Left, summary plots of mean normalized EPSP slope ± s.e.m. Right, cumulative histograms for average normalized EPSP slope during STP and LTP. a, Robust STP and LTP following HFS in myc–SEP–GluA2-replacement cells following NeutrAvidin pre-treatment. b, Rundown of basal transmission by 0.5 μM intracellular TeTx during pseudo HFS recordings of bAP–SEP–GluA2-replacement cells. c, Absent potentiation following HFS in bAP–SEP–GluA2-replacement cells when NeutrAvidin pre-treatment is combined with intracellular 500 μM N-ethylmaleimide (NEM). Statistical significance was assessed by repeated-measures ANOVA (a–c) ns, not significant; *P < 0.05; **P < 0.01.
Extended Data Figure 4 Presynaptic plasticity controls for NeutrAvidin crosslinking of bAP–SEP–GluA2.
a, NeutrAvidin has no effect on paired-pulse ratio (PPR) of: the slope of AMPA-mediated EPSPs evoked at 50 ms intervals in untransfected CA1 pyramidal neurons (a); the amplitude of NMDA-mediated EPSCs evoked at 50 ms intervals in bAP–SEP–GluA2-replacement cells (b). All bar graphs show means ± s.e.m. Statistical significance was assessed by unpaired t-tests; ns, not significant.
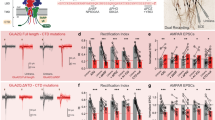
Extended Data Figure 5 Statistical comparison between all the different treatments for STP and LTP.
Bar graph summarizing statistical comparison of the data for the manipulations in Fig. 2a–e, Extended Data Figs 2b, c and 3a–c. Different AMPAR trafficking manipulations have distinct effects on synaptic potentiation. The results demonstrate that HFS-dependent STP is only significantly different from control when surface diffusion of existing surface AMPARs is prevented. By contrast, HFS-dependent LTP is significantly different only for manipulations that prevent the delivery of newly exocytosed receptors. Bar graph shows marginal means and error bars are least significant difference (LSD). Data points for all conditions are plotted in the cumulative histograms of Fig. 2a–e, Extended Data Figs 2b, c and Fig. 3a–c. Statistical significance was assessed by two-way repeated-measures ANOVA with Benjamini–Hochberg post hoc tests. ns, not significant; *P < 0.05; **P < 0.01; ***P < 0.001.
Extended Data Figure 6 Control experiments for antibody-mediated crosslink of AMPA receptors in cultured hippocampal neurons.
a Anti-GluA2 IgG (bivalent) but not Fab (monovalent) prevents normal FRAP of SEP–GluA2 in spines. Graphs show ensemble grand mean FRAP curves, fits and standard error bands for experiments using transfected cultures pre-treated for half an hour with 80 mg l−1 anti-GluA2 IgG clone 15F1 (bottom), 80 mg l−1 anti-GluA2 Fab clone 15F1 (middle) or vehicle (top). b, c, Relative frequency histogram of log-transformed diffusion coefficients (D, left) and bar graph of percentage mobile fractions obtained from single-particle tracking (SPT) experiments. Number of cells in parentheses. b, U-PAINT single-particle tracking of endogenous GluA2 in the presence or absence of anti-GluA2 IgG clone 14B11. c, PALM single-particle tracking of expressed mGluR5–mEOS in the presence or absence of anti-GluA2 IgG clone 14B11. Bar graphs show means ± s.e.m. Statistical significance was assessed by unpaired t-tests (b, c). ns, not significant; ***P < 0.001.
Extended Data Figure 7 No detectable effect of incubation with crosslinking anti-GluA2 IgG on basal endocytosis or phosphorylation of GluA1-containing AMPA receptors.
a, Schematic of the experimental protocol performed on DIV 17 cultured hippocampal neurons and data summary of fluorescence (normalized to the mean fluorescence at time zero) for anti-GluA1 antibody feeding for 30 min following 15 min crosslink by 10 μg ml−1 anti-GluA2 IgG (clone 15F1). Note that most GluA1 AMPA receptors in pyramidal neurons exist as GluA1/2 heteromers. Similar results were obtained from two experiments and combined, where the control was either no antibody or anti-GFP. The images are all scaled the same. b, Schematic of experimental protocol (top), images of example western blots (middle) and data for phosphorylation at GluA1 serine 845 and 831 after 15 min crosslink by 10 μg ml−1 anti-GluA2 IgG (clone 15F1) or control IgG (anti-GFP) (bottom). Phosphorylation was unaffected by the crosslink manipulation. c, Schematic of experimental chemical LTP (cLTP) protocol (top), images of example western blots (middle) and data for phosphorylation at GluA1 serine 845 after 15 min crosslink by 10 μg ml−1 anti-GluA2 IgG (clone 15F1) or control IgG (anti-GFP) followed by cLTP or control treatment (bottom). The crosslink manipulation had little impact on S845 phosphorylation induced by cLTP. We achieved similar phosphorylation results for AMPARs isolated by surface biotinylation and eluting from streptavidin beads. Bar graphs show means ± s.e.m. Note that in b and c the data points for each experiment were re-centred on the grand mean; thus the error bars approximate within-experiment s.e.m. Black arrowheads adjacent to the molecular weight (MW) lane in b and c denote the 95 kDa size marker. Statistical significance was assessed by mixed model nested ANOVA (a), two-way ANOVA without interaction (b) or two-way repeated-measures ANOVA. ns, not significant; ***P < 0.001. For gel source data, see Supplementary Fig. 1.
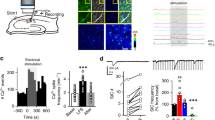
Extended Data Figure 8 Effect of crosslinking AMPA receptors on synaptic potentiation and basal transmission in acute hippocampal slices.
a, Schematic diagram illustrating the protocol for pre-injection antibody crosslink experiments in acute slices. b, Summary plots of mean normalized field EPSP slope (top) and paired-pulse ratio (PPR, 200 ms interval) of the slope (bottom) ± s.e.m. c, Input–output curves of the field EPSP slope are unaffected by antibody infusion. The fibre volley varied linearly over the range of stimulation intensities (data not shown). d, Input–output curves of evoked NMDAR-mediated EPSCs are unaffected by antibody infusion. e, Spontaneous EPSC frequency (left) and amplitude (right) are unaffected by antibody infusion. f, NMDA:AMPA ratios are unaffected by antibody infusion. ANOVA on log10(ratio NMDA:AMPA), F2,54 = 0.53, P = 0.5942. Numbers in brackets indicate the number of cells (f) or the number of slices (c–e), where measurements from whole-cell recordings within the same slice were averaged (d, e). Bar graphs show means ± s.e.m. Statistical significance was assessed by one-way ANCOVA (c, d) or one-way ANOVA (e, f). ns, not significant.
Supplementary information
Supplementary Information
This file contains Figures Statistics for Figures 1-4 and Extended Data Figures 1-8. (PDF 346 kb)
Rights and permissions
About this article
Cite this article
Penn, A., Zhang, C., Georges, F. et al. Hippocampal LTP and contextual learning require surface diffusion of AMPA receptors. Nature 549, 384–388 (2017). https://doi.org/10.1038/nature23658
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nature23658
This article is cited by
-
Effects of the AMPAR Antagonist, Perampanel, on Cognitive Function in Rats Exposed to Neonatal Iron Overload
Molecular Neurobiology (2024)
-
Effects of DeSUMOylated Spastin on AMPA Receptor Surface Delivery and Synaptic Function Are Enhanced by Phosphorylating at Ser210
Molecular Neurobiology (2024)
-
Mitochondrial impairment and synaptic dysfunction are associated with neurological defects in iPSCs-derived cortical neurons of MERRF patients
Journal of Biomedical Science (2023)
-
Enhanced AMPAR-dependent synaptic transmission by S-nitrosylation in the vmPFC contributes to chronic inflammatory pain-induced persistent anxiety in mice
Acta Pharmacologica Sinica (2023)
-
Neuronal network activity and connectivity are impaired in a conditional knockout mouse model with PCDH19 mosaic expression
Molecular Psychiatry (2023)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.