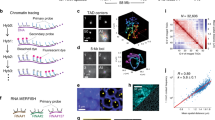
Abstract
The folding of genomic DNA from the beads-on-a-string-like structure of nucleosomes into higher-order assemblies is crucially linked to nuclear processes. Here we calculate 3D structures of entire mammalian genomes using data from a new chromosome conformation capture procedure that allows us to first image and then process single cells. The technique enables genome folding to be examined at a scale of less than 100 kb, and chromosome structures to be validated. The structures of individual topological-associated domains and loops vary substantially from cell to cell. By contrast, A and B compartments, lamina-associated domains and active enhancers and promoters are organized in a consistent way on a genome-wide basis in every cell, suggesting that they could drive chromosome and genome folding. By studying genes regulated by pluripotency factor and nucleosome remodelling deacetylase (NuRD), we illustrate how the determination of single-cell genome structure provides a new approach for investigating biological processes.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
Change history
05 April 2017
The graph for chromosome 14 in Extended Data Fig. 6 was corrected.
References
Cremer, T. et al. The 4D nucleome: Evidence for a dynamic nuclear landscape based on co-aligned active and inactive nuclear compartments. FEBS Lett. 589 (20 Pt A), 2931–2943 (2015)
Bickmore, W. A. & van Steensel, B. Genome architecture: domain organization of interphase chromosomes. Cell 152, 1270–1284 (2013)
Lieberman-Aiden, E. et al. Comprehensive mapping of long-range interactions reveals folding principles of the human genome. Science 326, 289–293 (2009)
Nora, E. P. et al. Spatial partitioning of the regulatory landscape of the X-inactivation centre. Nature 485, 381–385 (2012)
Dixon, J. R. et al. Topological domains in mammalian genomes identified by analysis of chromatin interactions. Nature 485, 376–380 (2012)
Sexton, T. et al. Three-dimensional folding and functional organization principles of the Drosophila genome. Cell 148, 458–472 (2012)
Rao, S. S. et al. A 3D map of the human genome at kilobase resolution reveals principles of chromatin looping. Cell 159, 1665–1680 (2014)
Zuin, J. et al. Cohesin and CTCF differentially affect chromatin architecture and gene expression in human cells. Proc. Natl Acad. Sci. USA 111, 996–1001 (2014)
Phillips-Cremins, J. E. et al. Architectural protein subclasses shape 3D organization of genomes during lineage commitment. Cell 153, 1281–1295 (2013)
Kalhor, R., Tjong, H., Jayathilaka, N., Alber, F. & Chen, L. Genome architectures revealed by tethered chromosome conformation capture and population-based modeling. Nat. Biotechnol. 30, 90–98 (2011)
Tjong, H. et al. Population-based 3D genome structure analysis reveals driving forces in spatial genome organization. Proc. Natl Acad. Sci. USA 113, E1663–E1672 (2016)
Naumova, N. et al. Organization of the mitotic chromosome. Science 342, 948–953 (2013)
Foti, R. et al. Nuclear architecture organized by Rif1 underpins the replication-timing program. Mol. Cell 61, 260–273 (2016)
Wang, S. et al. Spatial organization of chromatin domains and compartments in single chromosomes. Science 353, 598–602 (2016)
Julienne, H., Zoufir, A., Audit, B. & Arneodo, A. Human genome replication proceeds through four chromatin states. PLOS Comput. Biol. 9, e1003233 (2013)
Peric-Hupkes, D. et al. Molecular maps of the reorganization of genome-nuclear lamina interactions during differentiation. Mol. Cell 38, 603–613 (2010)
Meuleman, W. et al. Constitutive nuclear lamina-genome interactions are highly conserved and associated with A/T-rich sequence. Genome Res. 23, 270–280 (2013)
van Koningsbruggen, S. et al. High-resolution whole-genome sequencing reveals that specific chromatin domains from most human chromosomes associate with nucleoli. Mol. Biol. Cell 21, 3735–3748 (2010)
Kind, J. et al. Single-cell dynamics of genome-nuclear lamina interactions. Cell 153, 178–192 (2013)
Dunn, S. J., Martello, G., Yordanov, B., Emmott, S. & Smith, A. G. Defining an essential transcription factor program for naïve pluripotency. Science 344, 1156–1160 (2014)
Therizols, P. et al. Chromatin decondensation is sufficient to alter nuclear organization in embryonic stem cells. Science 346, 1238–1242 (2014)
Krüger, A. V. et al. Comprehensive single cell-resolution analysis of the role of chromatin regulators in early C. elegans embryogenesis. Dev. Biol. 398, 153–162 (2015)
Sofueva, S. et al. Cohesin-mediated interactions organize chromosomal domain architecture. EMBO J. 32, 3119–3129 (2013)
Giorgetti, L. et al. Predictive polymer modeling reveals coupled fluctuations in chromosome conformation and transcription. Cell 157, 950–963 (2014)
Naughton, C. et al. Transcription forms and remodels supercoiling domains unfolding large-scale chromatin structures. Nat. Struct. Mol. Biol. 20, 387–395 (2013)
Kouzine, F. et al. Transcription-dependent dynamic supercoiling is a short-range genomic force. Nat. Struct. Mol. Biol. 20, 396–403 (2013)
Guo, Y. et al. CRISPR inversion of CTCF sites alters genome topology and enhancer/promoter function. Cell 162, 900–910 (2015)
Sanborn, A. L. et al. Chromatin extrusion explains key features of loop and domain formation in wild-type and engineered genomes. Proc. Natl Acad. Sci. USA 112, E6456–E6465 (2015)
Fudenberg, G. et al. Formation of chromosomal domains by loop extrusion. Cell Reports 15, 2038–2049 (2016)
Lengronne, A. et al. Cohesin relocation from sites of chromosomal loading to places of convergent transcription. Nature 430, 573–578 (2004)
Eagen, K. P., Hartl, T. A. & Kornberg, R. D. Stable chromosome condensation revealed by chromosome conformation capture. Cell 163, 934–946 (2015)
Zhimulev, I. F. et al. Genetic organization of interphase chromosome bands and interbands in Drosophila melanogaster. PLoS One 9, e101631 (2014)
Minajigi, A. et al. Chromosomes. A comprehensive Xist interactome reveals cohesin repulsion and an RNA-directed chromosome conformation. Science 349, aab2276 (2015)
Giorgetti, L. et al. Structural organization of the inactive X chromosome in the mouse. Nature 535, 575–579 (2016)
de Wit, E. et al. The pluripotent genome in three dimensions is shaped around pluripotency factors. Nature 501, 227–231 (2013)
Wei, Z. et al. Klf4 organizes long-range chromosomal interactions with the Oct4 locus in reprogramming and pluripotency. Cell Stem Cell 13, 36–47 (2013)
Denholtz, M. et al. Long-range chromatin contacts in embryonic stem cells reveal a role for pluripotency factors and polycomb proteins in genome organization. Cell Stem Cell 13, 602–616 (2013)
Reynolds, N. et al. NuRD suppresses pluripotency gene expression to promote transcriptional heterogeneity and lineage commitment. Cell Stem Cell 10, 583–594 (2012)
Zhang, W. et al. The nucleosome remodeling and deacetylase complex NuRD is built from preformed catalytically active sub-modules. J. Mol. Biol. 428, 2931–2942 (2016)
Zhang, Y. et al. Spatial organization of the mouse genome and its role in recurrent chromosomal translocations. Cell 148, 908–921 (2012)
Murakami, K. et al. NANOG alone induces germ cells in primed epiblast in vitro by activation of enhancers. Nature 529, 403–407 (2016)
Acknowledgements
We thank A. Riddell for cell sorting, P. Humphreys for confocal microscopy, A. Peter Gunnarson for the density mapping software, the CRUK Cambridge Institute for DNA sequencing, T. Nagano and P. Fraser for processing the preliminary haploid mouse ES cells, and W. Dean, S. Schoenfelder and S. Wingett for advice. We thank the Wellcome Trust (082010/Z/07/Z), the EC FP7 4DCellFate project (277899) and the MRC (MR/M010082/1) for financial support.
Author information
Authors and Affiliations
Contributions
D.L., S.B. and Y.C. developed the protocol and carried out imaging/Hi-C processing. T.J.S. developed the software with assistance from L.P.A., W.B. and K.J.W. A.O’S.-K., J.C., M.R. and B.H. carried out the CHD4/MBD3 depletion experiments, associated RNA-seq and ChIP–seq, and created the mEos3.2-Halo tagged ES cell lines. M.L. and A.W. provided the initial samples of haploid mouse ES cells. S.F.L., M.G.S.P. and D.K. designed and built the microscope. L.M., M.S. and L.D.C. carried out ChIP–seq and RNA-seq experiments, while A.J.F., E.B. and B.L. carried out bioinformatics analysis. T.J.S. and E.D.L. designed experiments, analysed the results and wrote the manuscript with contributions from all the other authors.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Additional information
Reviewer Information Nature thanks W. Huber and the other anonymous reviewer(s) for their contribution to the peer review of this work.
Extended data figures and tables
Extended Data Figure 1 Quality control for Hi-C processing and 3D structure calculation.
a, Comparison of 3D images of CENP-A in haploid mouse ES cell nuclei, expressing mEos3.2-tagged CENP-A and tandem infrared fluorescent protein (iRFP)-tagged histone H2B, with their corresponding white-light images. b, Comparison of three single-cell Hi-C contact maps (above the diagonal; contacts coloured red, yellow and blue) with the population Hi-C map (below the diagonal). c, An analysis of the accuracy and precision of the 100-kb structure calculation procedure for cell 1. The graphs show how the global (dis)similarity of structures is affected by: the total number of contacts (left); the number of inter-chromosomal contacts (middle); and the number of random noise contacts (right). Mean r.m.s.d. values for all pairs of conformations ± s.e.m. are shown for: the precision within ensembles arising from ten re-calculations using the same contacts (red); the variation across ensembles that arises from different random resampling (blue); and, as a measure of accuracy, the similarity to the best ensemble of structures (yellow). d, An example of a structure calculation carried out using either a single dataset, or after randomly merging 50% of the data from two different cells. Strongly violated experimental restraints (>4 particle radii apart) are shown in red. The plot (right) shows the probability of any two particles connected by an experimental restraint being violated to different degrees. e, Left, the structure of chromosome 1 from cell 6, where part of the chromosome lies at the opposite side of the genome structure, with no intermediate chromosome folding, illustrating the presence of a chromosomal break or recombination event. Right, the contact map shows that there are no contacts from the disconnected region to any other part of chromosome 1, but clear contacts to chromosomes 3 and 7. f, An example of an attempted calculation of the haploid genome structure for a cell containing a duplicated chromosome 2 shows many violations of the experimental restraints for that chromosome and a much more compacted structure (here compared with chromosomes 1 and 3). The structures are coloured according to position in the chromosome sequence from red to purple (centromere to telomere).
Extended Data Figure 2 Validation and analysis of single-cell contacts.
a, Structure of the entire haploid mouse ES cell genome from cells 2 to 8. The structural ensemble is represented by five superimposed conformations from repeat calculations, and is shown in three different orientations (after rotation through 90° relative to each other), with the chromosomes coloured according to their position in the chromosome sequence from red to purple (centromere to telomere). b, Correspondence between the distribution of Hi-C contacts (both cis and trans), violations of the distance restraints in the 3D structures, and DNA replication timing13 for a representative chromosome (chromosome 12). c, Left, log-scale plots of contact probability (Pcont) against sequence separation (S). The slopes for a power law relationship (Pcont ∝ Sα) in which α is either −1.0 or −1.5 are also indicated. Data are shown for the combined single-cell Hi-C contact data, for all of the non-sequential particles that are close to each other in the structures (<2 particle radii apart), and for the population Hi-C data. Right, the distribution in the number of intra-chromosomal (cis) or inter-chromosomal (trans) contacts between 100-kb regions in the single-cell Hi-C data are shown for both the A and B compartments. d, Correlation of gene expression levels (left), and hierarchically clustered heat maps showing the pairwise enrichment of ChIP–seq peak overlaps between haploid and diploid ES cells (centre), and Nanog ChIP–seq peak overlaps between haploid and diploid ES cells used in this study, as well as that previously published from diploid ES cells (right)41.
Extended Data Figure 3 Chromosome interactions.
a, Violin plot showing the proportion of each chromosome that intermingles with other chromosomes. b, Pairwise comparison of the chromosome structure in different cells by r.m.s.d. analysis. Four models of chromosome 9 from a selection of different cells are shown, coloured according to the chromosome sequence (from red to purple, centromere to telomere), together with a table showing the r.m.s.d. values between the chromosomal 3D coordinates for each cell (bottom). c, Further cross-sections through the structures of haploid genomes from cells 3–8 through the structures of haploid genomes (see Fig. 2e), coloured according to: whether the sequence is in the A or B compartment (top); whether the sequence is part of a cLAD or contains highly expressed genes (coloured yellow and blue, respectively) (centre); and the identity of the chromosomes (bottom). In each case, an ensemble of five superimposed conformations arising from repeat calculations starting from different randomly generated sets of coordinates is shown. d, An analysis of the genome depth of various chromatin class categories, determined by k-means clustering of 100-kb segments according to the presence of histone H3 ChIP–seq data15. The active class is associated with H3K4me3, Polycomb with H3K27me3, the inactive class with H3K9me3, and null denotes the remainder. Left, the probability distribution for each of the categories at different normalized nucleus depths. Right, the divergence of the probability distribution for each category from the whole-genome average. Data are shown for the genome structures of all cells. e, An analysis of the genome depth for regions with differing levels of gene expression, as measured by nuclear RNA-seq. Here, RNA-seq signal peaks were ranked and split into five classes. As in d, the probability distribution for each class with regard to genome depth is shown (left), together with the divergence of each distribution from the genome as a whole (right). f, Further comparisons of the structure of chromosome 3 from different cells, coloured according to whether the sequence is part of the cLAD domains (yellow), with the positions of highly expressed genes indicated by the blue rings (larger circles indicate higher expression).
Extended Data Figure 4 Relationship between genome folding and gene expression.
a, Calculation of 3D spatial clustering compared to a random hypothesis in which the same data were circularly permuted around the sequence, and repeating the calculations, using the same structure. Two examples, with strong (Klf4/H3K4me1) and weaker (Nanog/H3K27me3) spatial co-localization compared to random, are shown. b, The enrichment in spatial density (after removal of any clustering expected from their being located in the same chromosome sequence) of histone H3 with various post-translational modifications and selected pluripotency factors as determined by ChIP–seq data. The enrichment is calculated over all cells as the Kullback–Liebler divergence of the normalized spatial density distribution from a random, circularly permuted, expectation (see Supplementary Methods for more details), and the data are presented in hierarchical cluster order, grouping the most similar datasets together. c, Box and whisker plots showing enhancer, promoter and repetitive sequence content (bottom), and the enrichment in spatial density of different types of enhancer, promoter and repetitive sequence (top), after the data have been divided into ten groups based on increasing distance from the nearest inter-chromosomal interface. Whiskers represent the tenth and ninetieth percentiles, boxes represent the range from the twenty-fifth to the seventy-fifth percentile, and outliers are shown as dots. Mean and median values are shown by black crosses and bars, respectively. The R values are the Pearson’s correlation coefficient on the underlying, unranked data. d, Plots of the level of gene expression as measured by the nuclear RNA-seq signal within 1-Mb regions against distance from the nearest inter-chromosomal interface (left) and the outer surface of the A compartment (right). e, Examples of inter-chromosomal interfaces from two different cells in which the chromosomes are coloured increasingly bright red for higher enrichment in the density of gene expression, compared to what would be expected for a given sequence separation. The remainder of the two chromosomes is coloured grey, and the positions of promoters are indicated by blue circles. The same views are shown with the two different chromosomes coloured yellow and blue (top), or with their regions in the A and B compartments coloured blue and red (bottom).
Extended Data Figure 5 Chromosome folding into compartments, TADs and loops.
a, A contact map showing the population Hi-C data for chromosome 12 with TADs identified using the directionality index5 as blue squares. On the left and at the bottom, data tracks are shown identifying the A and B compartments (in blue and red, respectively), and highly expressed genes (in magenta). b, Further comparisons (see Fig. 4b) showing the structures (and their variability) of two B compartment TADs either side of a highly expressed gene(s) in a short region of A compartment (top), or at a boundary between the A and B compartments (bottom). Ensembles of five superimposed conformations, from repeat calculations using the same experimental data, are shown with pairs of TADs highlighted and coloured according to whether they are in the A or B compartments (blue and red, respectively), with white indicating a transitional segment (between A and B). TAD boundaries are marked by asterisks. c, Scatter plots of the mean radius of gyration for 1-Mb regions of genome structure compared to the average number of single-cell Hi-C contacts, within the same region, considering a 1-Mb sliding analysis window. Data are shown for all genome structures and split according to cis (left) and trans (right) contacts. d, Structure of chromosome 12, with the A compartment coloured blue and positions of CTCF/cohesin loops identified previously7 indicated by dotted red lines. The pie chart shows the numbers of loops between sequences in the A and B compartments.
Extended Data Figure 6 Chromosome folding into TADs.
Bar charts of the mean  values of TADs identified using the directionality index5 for all the different chromosomes. The data are mean values over all structure conformations, scaled according to TAD size, and presented as quantile values for the chromosome. The fiftieth percentile value corresponds to the central grey line. Values below and above this are coloured blue and red respectively. TADs that contain both regions of early replication timing (above the ninetieth percentile) and moderate restraint violation (see Extended Data Fig. 2b) are excluded from the calculation. The errors in the
values of TADs identified using the directionality index5 for all the different chromosomes. The data are mean values over all structure conformations, scaled according to TAD size, and presented as quantile values for the chromosome. The fiftieth percentile value corresponds to the central grey line. Values below and above this are coloured blue and red respectively. TADs that contain both regions of early replication timing (above the ninetieth percentile) and moderate restraint violation (see Extended Data Fig. 2b) are excluded from the calculation. The errors in the  are the percentiles ± the s.e.m. Values for multiple cells are presented in hierarchical cluster order, grouping the most similar cells together.
are the percentiles ± the s.e.m. Values for multiple cells are presented in hierarchical cluster order, grouping the most similar cells together.
Extended Data Figure 7 Chromosome folding into loops.
A genome-wide analysis illustrating whether CTCF/cohesin loops7 could be formed in the different single cells, in each chromosome. A black square indicates that the two boundaries in the loop could interact, a white square indicates that the two relevant particles are too far apart in the structure. The loop boundary separation, in particles, is shown along the x axis. The bar chart across the top shows the probability, for each loop, of random particles (pairs with the same sequence separation) forming the same number of contacts, or better. The probability of choosing a set of loop boundary points, which interact more frequently than we observed is 0.00072 (see Supplementary Methods).
Extended Data Figure 8 Understanding the nature of gene networks in mouse ES cells.
a, Structures of cells 2–8 illustrating the interactions identified between the Nanog gene and other regions of the genome by population 4C (ref. 34). Chromosome 6 is coloured blue, with the position of the Nanog gene highlighted in yellow, and the remainder of the chromosomes are coloured grey. Interacting positions in the genome are indicated by red circles. b, Heat map showing the number of times a particular interaction is detected between two of the 4C Nanog gene-interacting points35. c, Heat map showing the number of times a particular interaction is detected between two of the 4C Pou5f1 gene-interacting points36. In b and c, the interaction points are presented in hierarchical order, grouping the regions that show the most interactions together. d, 2D single-molecule tracking using photo-activated light microscopy in live mouse ES cells shows clustering of CHD4 and MBD3. In both cases, a heat map of a single cell is shown in which the pixels have been colour-coded according to the density of molecules detected in that region.
Supplementary information
Supplementary Information
This file contains a description of the protocols for cell culture, imaging and single-cell Hi-C, as well as the procedures used for processing the Hi-C data, and calculating/analysing the 3D structures. Details of how to download the software are also provided. (PDF 903 kb)
Five superimposed 3D structures of the intact mouse ES cell genome for Cell 1
An expanding view of the chromosomes, which are coloured differently, is shown – see Figs. 1c, 2c for more details. (MOV 40598 kb)
Five superimposed 3D structures of chromosome 10 from the intact mouse ES cell genome for Cell 1.
Five superimposed 3D structures of chromosome 10 from the intact mouse ES cell genome for Cell 1, coloured from red through to purple (centromere to telomere) – see Fig. 1c for more details. (MOV 9700 kb)
Five superimposed 3D structures of the intact mouse ES cell genome for Cell 1
Five superimposed 3D structures of the intact mouse ES cell genome for Cell 1, with the chromosomes coloured from red through to purple (centromere to telomere) – see Fig. 2a for more details. (MOV 19216 kb)
3D structure of the intact mouse ES genome for Cell 1
3D structure of the intact mouse ES genome for Cell 1, with expanding views of the spatial distribution of the A (blue) and B (red) compartments – see Figs. 2c,d,e for more details. (MOV 41600 kb)
3D structure of chromosome 10 from the intact mouse ES cell genome for Cell 1
3D structure of chromosome 10 from the intact mouse ES cell genome for Cell 1, illustrating the way the chromosome contributes to the A/B compartments – see Fig. 2f for more details. (MOV 10047 kb)
3D structure of the intact mouse ES genome for Cell 1
3D structure of the intact mouse ES genome for Cell 1, with expanding views of the spatial distribution of the constitutive lamin-associated domains (cLADs) (yellow) and regions containing highly expressed genes (blue) – see Fig. 2e for more details. (MOV 45994 kb)
3D structure of chromosome 10 from the intact mouse ES cell genome for Cell 1
3D structure of chromosome 10 from the intact mouse ES cell genome for Cell 1, illustrating the way the chromosome contributes to the spatial distribution of the constitutive lamin-associated domains (cLADs) (yellow) and regions containing highly expressed genes (blue) – see Fig. 2g for more details. (MOV 9778 kb)
Close up views of the 3D structures of two B compartment TADs (Region 1 in Fig. 5a for Cell 4).
The TADs are coloured according to whether they are in the A (blue) or B (red) compartments, with white indicating a transitional segment (between A and B). (MOV 16596 kb)
Close up views of the 3D structures of two B compartment TADs (Region 1 in Fig. 5a for Cell 5).
The TADs are coloured according to whether they are in the A (blue) or B (red) compartments, with white indicating a transitional segment (between A and B). (MOV 17252 kb)
Close up views of the 3D structures of TADs either side of an A/B compartment boundary (Region 2 in Fig. 5a for Cell 4).
The TADs are coloured according to whether they are in the A (blue) or B (red) compartments, with white indicating a transitional segment (between A and B). (MOV 27338 kb)
Close up views of the 3D structures of TADs either side of an A/B compartment boundary (Region 2 in Fig. 5a for Cell 5).
The TADs are coloured according to whether they are in the A (blue) or B (red) compartments, with white indicating a transitional segment (between A and B). (MOV 17872 kb)
Rights and permissions
About this article
Cite this article
Stevens, T., Lando, D., Basu, S. et al. 3D structures of individual mammalian genomes studied by single-cell Hi-C. Nature 544, 59–64 (2017). https://doi.org/10.1038/nature21429
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nature21429
This article is cited by
-
DiffDomain enables identification of structurally reorganized topologically associating domains
Nature Communications (2024)
-
Computational methods for analysing multiscale 3D genome organization
Nature Reviews Genetics (2024)
-
Pan-evolutionary and regulatory genome architecture delineated by an integrated macro- and microsynteny approach
Nature Protocols (2024)
-
Conserved chromatin and repetitive patterns reveal slow genome evolution in frogs
Nature Communications (2024)
-
Does multi-way, long-range chromatin contact data advance 3D genome reconstruction?
BMC Bioinformatics (2023)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.