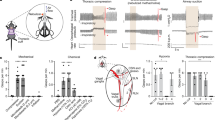
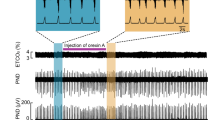
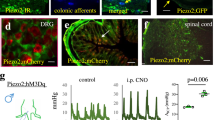
Abstract
Respiratory dysfunction is a notorious cause of perinatal mortality in infants and sleep apnoea in adults, but the mechanisms of respiratory control are not clearly understood. Mechanical signals transduced by airway-innervating sensory neurons control respiration; however, the physiological significance and molecular mechanisms of these signals remain obscured. Here we show that global and sensory neuron-specific ablation of the mechanically activated ion channel Piezo2 causes respiratory distress and death in newborn mice. Optogenetic activation of Piezo2+ vagal sensory neurons causes apnoea in adult mice. Moreover, induced ablation of Piezo2 in sensory neurons of adult mice causes decreased neuronal responses to lung inflation, an impaired Hering–Breuer mechanoreflex, and increased tidal volume under normal conditions. These phenotypes are reproduced in mice lacking Piezo2 in the nodose ganglion. Our data suggest that Piezo2 is an airway stretch sensor and that Piezo2-mediated mechanotransduction within various airway-innervating sensory neurons is critical for establishing efficient respiration at birth and maintaining normal breathing in adults.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Rhoades, R. A. & Bell, D. R. Medical Physiology: Principles for Clinical Medicine 3rd edn, 328 (Lippincott Williams & Wilkins, 2009)
Schelegle, E. S. & Green, J. F. An overview of the anatomy and physiology of slowly adapting pulmonary stretch receptors. Respir. Physiol. 125, 17–31 (2001)
Lee, L. Y. & Yu, J. Sensory nerves in lung and airways. Compr. Physiol. 4, 287–324 (2014)
Zhang, J. W., Walker, J. F., Guardiola, J. & Yu, J. Pulmonary sensory and reflex responses in the mouse. J. Appl. Physiol. 101, 986–992 (2006)
Carr, M. J. & Undem, B. J. Bronchopulmonary afferent nerves. Respirology 8, 291–301 (2003)
Kaczyn´ska, K. & Szereda-Przestaszewska, M. Nodose ganglia-modulatory effects on respiration. Physiol. Res. 62, 227–235 (2013)
Belvisi, M. G. Overview of the innervation of the lung. Curr. Opin. Pharmacol. 2, 211–215 (2002)
Alexandrova, N. P., Donina, Z. A. & Danilova, G. A. Effect of central hypervolemia on respiratory function. J. Physiol. Pharmacol. 58 (Suppl. 5), 9–15 (2007)
Chang, R. B., Strochlic, D. E., Williams, E. K., Umans, B. D. & Liberles, S. D. Vagal sensory neuron subtypes that differentially control breathing. Cell 161, 622–633 (2015)
Turgeon, B. & Meloche, S. Interpreting neonatal lethal phenotypes in mouse mutants: insights into gene function and human diseases. Physiol. Rev. 89, 1–26 (2009)
Rabbette, P. S. & Stocks, J. Influence of volume dependency and timing of airway occlusions on the Hering-Breuer reflex in infants. J. Appl. Physiol. 85, 2033–2039 (1998)
Wong, K. A. et al. Pulmonary vagal innervation is required to establish adequate alveolar ventilation in the newborn lamb. J. Appl. Physiol. 85, 849–859 (1998)
Hasan, S. U., Lalani, S. & Remmers, J. E. Significance of vagal innervation in perinatal breathing and gas exchange. Respir. Physiol. 119, 133–141 (2000)
Ranade, S. S., Syeda, R. & Patapoutian, A. Mechanically activated ion channels. Neuron 87, 1162–1179 (2015)
Ranade, S. S. et al. Piezo2 is the major transducer of mechanical forces for touch sensation in mice. Nature 516, 121–125 (2014)
Coste, B. et al. Piezo1 and Piezo2 are essential components of distinct mechanically activated cation channels. Science 330, 55–60 (2010)
Woo, S. H. et al. Piezo2 is the principal mechanotransduction channel for proprioception. Nat. Neurosci. 18, 1756–1762 (2015)
Schrenk-Siemens, K. et al. PIEZO2 is required for mechanotransduction in human stem cell-derived touch receptors. Nat. Neurosci. 18, 10–16 (2015)
Dubin, A. E. et al. Inflammatory signals enhance piezo2-mediated mechanosensitive currents. Cell Reports 2, 511–517 (2012)
Pei, L. et al. Thyroid hormone receptor repression is linked to type I pneumocyte-associated respiratory distress syndrome. Nat. Med. 17, 1466–1472 (2011)
Woo, S. H. et al. Piezo2 is required for Merkel-cell mechanotransduction. Nature 509, 622–626 (2014)
Pan, J., Copland, I., Post, M., Yeger, H. & Cutz, E. Mechanical stretch-induced serotonin release from pulmonary neuroendocrine cells: implications for lung development. Am. J. Physiol. Lung Cell. Mol. Physiol. 290, L185–L193 (2006)
Cutz, E., Pan, J., Yeger, H., Domnik, N. J. & Fisher, J. T. Recent advances and contraversies on the role of pulmonary neuroepithelial bodies as airway sensors. Semin. Cell Dev. Biol. 24, 40–50 (2013)
Smith, J. C., Abdala, A. P., Rybak, I. A. & Paton, J. F. Structural and functional architecture of respiratory networks in the mammalian brainstem. Phil. Trans. R. Soc. Lond. B 364, 2577–2587 (2009)
Koni, P. A. et al. Conditional vascular cell adhesion molecule 1 deletion in mice: impaired lymphocyte migration to bone marrow. J. Exp. Med. 193, 741–754 (2001)
Danielian, P. S., Muccino, D., Rowitch, D. H., Michael, S. K. & McMahon, A. P. Modification of gene activity in mouse embryos in utero by a tamoxifen-inducible form of Cre recombinase. Curr. Biol. 8, 1323–1326 (1998)
Scott, M. M., Williams, K. W., Rossi, J., Lee, C. E. & Elmquist, J. K. Leptin receptor expression in hindbrain Glp-1 neurons regulates food intake and energy balance in mice. J. Clin. Invest. 121, 2413–2421 (2011)
Nassenstein, C. et al. Phenotypic distinctions between neural crest and placodal derived vagal C-fibres in mouse lungs. J. Physiol. (Lond.) 588, 4769–4783 (2010)
Chai, Y. et al. Fate of the mammalian cranial neural crest during tooth and mandibular morphogenesis. Development 127, 1671–1679 (2000)
Li, B. Y., Glazebrook, P., Kunze, D. L. & Schild, J. H. KCa1.1 channel contributes to cell excitability in unmyelinated but not myelinated rat vagal afferents. Am. J. Physiol. Cell Physiol. 300, C1393–C1403 (2011)
Ruan, H. Z. & Burnstock, G. Localisation of P2Y1 and P2Y4 receptors in dorsal root, nodose and trigeminal ganglia of the rat. Histochem. Cell Biol. 120, 415–426 (2003)
Lee, K. Z. et al. Hypoglossal neuropathology and respiratory activity in pompe mice. Front. Physiol. 2, 31 (2011)
McGovern, A. E. et al. Evidence for multiple sensory circuits in the brain arising from the respiratory system: an anterograde viral tract tracing study in rodents. Brain Struct. Funct. 220, 3683–3699 (2015)
Parkes, M. J. Breath-holding and its breakpoint. Exp. Physiol. 91, 1–15 (2006)
Chesler, A. T. et al. The role of PIEZO2 in human mechanosensation. N. Engl. J. Med. 375, 1355–1364 (2016)
Delle Vedove, A. et al. Biallelic loss of proprioception-related PIEZO2 causes muscular atrophy with perinatal respiratory distress, arthrogryposis, and scoliosis. Am. J. Hum. Genet. 99, 1206–1216 (2016)
Coste, B. et al. Gain-of-function mutations in the mechanically activated ion channel PIEZO2 cause a subtype of distal arthrogryposis. Proc. Natl Acad. Sci. USA 110, 4667–4672 (2013)
Okubo, M. et al. A family of distal arthrogryposis type 5 due to a novel PIEZO2 mutation. Am. J. Med. Genet. A. 167A, 1100–1106 (2015)
Tryfon, S., Kontakiotis, T., Mavrofridis, E. & Patakas, D. Hering–Breuer reflex in normal adults and in patients with chronic obstructive pulmonary disease and interstitial fibrosis. Respiration 68, 140–144 (2001)
Nishino, T. Physiological and pathophysiological implications of upper airway reflexes in humans. Jpn J. Physiol. 50, 3–14 (2000)
Thach, B. T. The role of respiratory control disorders in SIDS. Respir. Physiol. Neurobiol. 149, 343–353 (2005)
Ramirez, J. M. et al. Central and peripheral factors contributing to obstructive sleep apneas. Respir. Physiol. Neurobiol. 189, 344–353 (2013)
Fox, E. A. et al. Neurotrophin-4 deficient mice have a loss of vagal intraganglionic mechanoreceptors from the small intestine and a disruption of short-term satiety. J. Neurosci. 21, 8602–8615 (2001)
Lu, Y. et al. The ion channel ASIC2 is required for baroreceptor and autonomic control of the circulation. Neuron 64, 885–897 (2009)
Sun, B., Li, Q., Dong, L. & Rong, W. Ion channel and receptor mechanisms of bladder afferent nerve sensitivity. Auton. Neurosci. 153, 26–32 (2010)
Metzger, R. J., Klein, O. D., Martin, G. R. & Krasnow, M. A. The branching programme of mouse lung development. Nature 453, 745–750 (2008)
Massey, C. A. et al. Isoflurane abolishes spontaneous firing of serotonin neurons and masks their pH/CO2 chemosensitivity. J. Neurophysiol. 113, 2879–2888 (2015)
Ramanantsoa, N. et al. Ventilatory response to hyperoxia in newborn mice heterozygous for the transcription factor Phox2b. Am. J. Physiol. Regul. Integr. Comp. Physiol. 290, R1691–R1696 (2006)
Barker, P. M. & Gatzy, J. T. Effect of gas composition on liquid secretion by explants of distal lung of fetal rat in submersion culture. Am. J. Physiol. 265, L512–L517 (1993)
Kwong, K. et al. Voltage-gated sodium channels in nociceptive versus non-nociceptive nodose vagal sensory neurons innervating guinea pig lungs. J. Physiol. (Lond.) 586, 1321–1336 (2008)
Dinh, Q. T. et al. Substance P expression in TRPV1 and trkA-positive dorsal root ganglion neurons innervating the mouse lung. Respir. Physiol. Neurobiol. 144, 15–24 (2004)
Chamberlin, N. L. Functional organization of the parabrachial complex and intertrigeminal region in the control of breathing. Respir. Physiol. Neurobiol. 143, 115–125 (2004)
Driessen, A. K., Farrell, M. J., Mazzone, S. B. & McGovern, A. E. The role of the paratrigeminal nucleus in vagal afferent evoked respiratory reflexes: a neuroanatomical and functional study in guinea pigs. Front. Physiol. 6, 378 (2015)
Bonham, A. C. & McCrimmon, D. R. Neurones in a discrete region of the nucleus tractus solitarius are required for the Breuer-Hering reflex in rat. J. Physiol. (Lond.) 427, 261–280 (1990)
Mortola, J. P. Respiratory Physiology of Newborn Mammals 24–37 (Johns Hopkins Univ. Press, 2001)
Woo, S. H., Lumpkin, E. A. & Patapoutian, A. Merkel cells and neurons keep in touch. Trends Cell Biol. 25, 74–81 (2015)
Vijayaraghavan, R. et al. Computer assisted recognition and quantitation of the effects of airborne chemicals acting at different areas of the respiratory tract in mice. Arch. Toxicol. 68, 490–499 (1994)
Hamelmann, E. et al. Noninvasive measurement of airway responsiveness in allergic mice using barometric plethysmography. Am. J. Respir. Crit. Care Med. 156, 766–775 (1997)
Acknowledgements
We thank D. Trajkovic for assistance with histology; M. Wood for assistance with electron microscopy; J. Yu for suggesting that we test the Hering–Breuer reflex; S. M. Cahalan, M. Petrus, J. Mathur, K. Marshall, S. Lee, T. Kawamura, J. Chen and P. Paolo Sanna for technical assistance; and M. Krasnow for discussions. This research was supported by NIH grants R01DE022358 to A.P. and R01HL132255, and a Giovanni Armenise-Harvard Foundation Grant to S.D.L. A.P. is an investigator of the Howard Hughes Medical Institute.
Author information
Authors and Affiliations
Contributions
A.G. characterized embryonic lungs in the laboratory of M. Krasnow. Z.Q. performed the initial characterization of Piezo2−/− mice. A.G.F. performed plethysmograph recordings and behavioural experiments to confirm Piezo2 knockdown. S.S.R. generated Piezo2fl/fl and Piezo2+/− mice. R.B.C. performed optogenetic experiments, whole vagus nerve electrophysiology recordings, and transducer-based Hering–Breuer reflex assessment in the S.D.L. laboratory. K.N. and S.-H.W. contributed equally to all other experiments. K.N., S.-H.W. and A.P. wrote the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Additional information
Reviewer Information Nature thanks M. Boehnke, J. M. Greally, B. Voight and the other anonymous reviewer(s) for their contribution to the peer review of this work.
Extended data figures and tables
Extended Data Figure 1 Characterization of lung development in Piezo2−/− mice.
a, b, Whole-mount staining for αSMA in the left lobe of lungs from E16.5 (a) and E18.5 (b) wild-type and Piezo2−/− mice. L1–L6, lateral branches of the left lobe. E16.5: wild-type n = 4, Piezo2−/− n = 3; E18.5: wild-type n = 4, Piezo2−/− n = 5. A normal conducting airway and vessel pattern was observed in Piezo2−/− lungs at late embryonic stages. c, d, Whole-mount immunostaining for E-cadherin and Muc-1 (c) or E-cadherin, T1α, and SpC (d) in left lobes from E18.5 wild-type and Piezo2−/− mice. Arrows indicate proximal (P) to distal (D) direction. Left panels, lower magnification; right panels, higher magnification of the distal region. Solid circles in right panels indicate alveolar progenitors in the distal region. Solid hexagons in left panels indicate nascent type I pneumocytes. Yellow dashed circles in left panels indicate nascent type II pneumocytes. A normal epithelial morphology and no defects in alveolar epithelial patterning and differentiation were observed Piezo2−/− lungs. e, Representative ultrastructures of wild-type and Piezo2−/− newborn lungs. Black arrows mark type I pneumocyte extensions. White dotted circles mark type II pneumocytes. White arrowheads within white dotted circles mark lamellar bodies. RBC, red blood cells. Samples from four mice per genotype were analysed. Normal morphology and similar abundances of type I and II pneumocytes, endothelial cells, red blood cells and surfactant proteins were observed in Piezo2−/− lungs compared to wild-type lungs. f, Wet-to-dry lung ratio 6 h after delivery at E18.5 to assess clearance of fetal pulmonary fluid. NS, statistically not significant. Unpaired Student’s t-test. Bars represent mean ± s.d. g, Per cent weight of postnatal day (P)0 lung, heart, and liver normalized to whole body weight. Bars represent mean ± s.e.m. NS, statistically not significant. Kruskal–Wallis nonparametric test (lung and liver) or one-way ANOVA (heart). Scale bars, 500 μm (a, b); 33 μm (c, d, left); 22 μm (c, d, right); 5 μm (e).
Extended Data Figure 2 Piezo2 expression in the respiratory system.
a, Co-immunostaining for GFP and Nefh in P0 Piezo2GFP reporter lung. Arrow, NEB. Smaller panels on right show a magnified view of NEB stained for GFP and CGRP (a marker of NEBs). b, c, GFP immunostaining in the jugular–nodose complex from P0 (b) and adult (c) Piezo2GFP reporter mice. Dotted line demarcates the boundary of the jugular–nodose complex. d, e, GFP immunostaining in P0 trigeminal ganglion (d) or adult thoracic DRG (e) from Piezo2GFP reporter mice. Dotted line in (d) demarcates the boundary of the trigeminal ganglion. f, Co-immunostaining for GFP and NeuN in a sagittal section of P0 Piezo2GFP brainstem. g, h, GFP and αBTX (a marker of neuromuscular junction) co-staining in P0 Piezo2GFP diaphragm (g) and intercostal muscles (h). i, Co-immunostaining for GFP and NeuN in adult Piezo2GFP lumbar spinal cord. Dotted circle indicates motor neuron localization. j, Co-immunostaining for GFP and NeuN in adult Piezo2GFP brainstem. Dotted circle marks dorsal nucleus of the vagus nerve, X. k, Co-immunostaining for GFP and Tuj1 in thoracic sympathetic ganglia from adult Piezo2GFP reporter mice. l, Schematic summary of Piezo2–GFP expression in the respiratory system. VII, facial nucleus; BötC, the Bötzinger complex; VRG, ventral respiratory group; RB, rib bone; Vt, ventricle. Scale bars, 20 μm (smaller panels on right in a), 100 μm (all other panels).
Extended Data Figure 3 Characterization of tdTomato expression in the respiratory system of Piezo2-GFP-IRES-Cre (Piezo2GFP);Ai9 reporter mice.
a, b, Immunostaining for PECAM1 (a) and CGRP (b) with tdTomato epifluorescence in postnatal lungs. Arrow in (b) indicates tdTomato+ nerve fibre innervating NEB. Dotted line in (b) demarcates the lung epithelium. c, tdTomato epifluorescence in P0 jugular–nodose complex. Dotted line in (c) demarcates the boundary of the jugular–nodose complex. d, tdTomato epifluorescence in adult thoracic DRG. e, f, tdTomato epifluorescence in adult nucleus of the solitary tract (NTS) (e) and in adult spinal trigeminal nucleus (Sp5) (f), where axons of nodose and jugular/trigeminal sensory neurons project, respectively52,53. Smaller panels on right in f show a magnified view of Sp5 with Nefh staining. g–i, PECAM1 immunostaining with tdTomato epifluorescence in P0 brainstem (g), adult diaphragm (h), and P0 intercostal muscle (i). j, PV immunostaining with tdTomato epifluorescence in postnatal lumbar spinal cord. Dotted circle marks motor neuron localization. k, Tuj1 immunostaining with tdTomato epifluorescence in adult thoracic sympathetic ganglia. l, ChAT immunostaining and tdTomato epifluorescence in tracheal parasympathetic ganglia from adult reporter mice. Scale bars, 25 μm (a, l, smaller panels on right in f), 20 μm (b), 100 μm (c–k).
Extended Data Figure 4 Characterization of tissue-specific Cre activities via Ai9 reporters.
a, b, tdTomato epifluorescence in P0 Tie2Cre;Ai9 lung with PECAM1 staining (a) or CGRP staining (b). c, tdTomato epifluorescence in P0 Tie2Cre;Ai9 jugular–nodose complex with PECAM1 staining. Dotted line demarcates the boundary of the jugular–nodose complex. d, tdTomato epifluorescence in adult Tie2Cre;Ai9 thoracic DRG. Dotted line demarcates the boundary of the DRG. e, tdTomato epifluorescence in P0 Tie2Cre;Ai9 trigeminal ganglion with Advillin staining. TdTomato signal co-localizes with PECAM1+ endothelial cells in a and c. f–i, tdTomato epifluorescence in P0 Phox2bCre;Ai9 lung with CGRP staining (f), P0 jugular–nodose complex with Advillin staining (g), adult thoracic DRG (h), and P0 trigeminal ganglion with Advillin staining (i). TdTomato signal is present in nodose ganglia (g), but absent in lung cells and NEBs (f), jugular ganglia (g), DRG (h), and trigeminal ganglia (i). Arrows in f indicate tdTomato+ vagal nerve fibre innervating the lung epithelium. Dotted line in h demarcates the boundary of DRG. j–m, tdTomato epifluorescence in P0 Wnt1Cre;Ai9 lung with CGRP staining (j), P0 jugular–nodose complex (k), adult thoracic DRG (l), and adult jugular–nodose complex with Piezo2 staining (m). m′, m′′, Higher magnification images of m. Arrows show tdTomato expression in both jugular neuronal cell bodies and satellite cells. Arrowheads show tdTomato expression only in satellite cells. TdTomato signal is present in neuronal cell bodies of jugular ganglia (k, m) and DRG (l), and satellite cells in the jugular–nodose complex and DRG (k–m), but absent in lung cells and NEBs (j) and neuronal cell bodies of nodose ganglia (k, m). TdTomato+ nerve fibres innervate the lung (j). Dotted lines indicate boundaries of the ganglia. VII + VIII, facial-acoustic complex. Scale bars, 50 μm (b), 12.5 μm (m′, m′′), 100 μm (all other panels).
Extended Data Figure 5 Characterization of Piezo2 knockdown in Piezo2 conditional knockout mice.
a, Characterization of Piezo2 knockdown by qRT–PCR using FACS-sorted CD31+ (or PECAM1+) lung cells from adult wild-type and Tie2Cre;Piezo2cKO mice. b, Piezo2 in situ hybridization in the jugular–nodose complex of adult wild-type and Phox2bCre;Piezo2cKO mice. Dotted circles mark nodose ganglia (N). c, qRT–PCR using DRG isolated from P0 wild-type and Wnt1Cre;Piezo2cKO pups. *P < 0.05, ***P < 0.001, ****P < 0.0001, unpaired Welch’s t-test for a, c. Data are presented as mean ± s.e.m. Scale bar, 100 μm.
Extended Data Figure 6 Optogenetic activation of Piezo2+ vagal sensory neurons in Piezo2GFP;lox-ChR2 mice.
a, A compound action potential response following brief optogenetic stimulation (blue lightning sign) of vagus nerve in Piezo2GFP;lox-ChR2 mice. b, A and C currents classified on the basis of corresponding peak area in the compound action potential. Dashed line: A–C ratio of 1. Data are presented as mean ± s.e.m. c, GFP, Nefh and IB4 co-staining in adult Piezo2GFP jugular–nodose complex. Red arrows indicate GPF+ Nefh+ IB4− cells; blue arrows indicate GFP+ Nefh− IB4+ cells. d, Percentage of Nefh or IB4 positive cells among GFP+ cells in adult Piezo2GFP jugular–nodose complex. e–g, State of respiratory trapping following optogenetic stimulation (50 Hz, 10 s) of vagus nerve in Piezo2GFP;lox-ChR2 mice. Representative trace showing changes in lung volume following optogenetic activation in Piezo2GFP;lox-ChR2 mice (e). Per cent change in total lung volume in Piezo2GFP;lox-ChR2 mice without and with light (f). The percentage of time in a high lung volume state (greater than mean volume during tidal breathing) in Piezo2GFP;lox-ChR2 mice without and with light (g). ***P < 0.001, ****P < 0.0001, paired t-test, mean ± s.e.m. h, Brainstem of Piezo2GFPmice with AAV-flex-tdTomato injection to the jugular–nodose complex. Sol, solitary tract; CC, central canal; AP, area postrema; L-NTS; ventral, lateral, ventrolateral, interstitial, and intermediate NTS subnuclei; M-NTS; dorsolateral, dorsomedial, medial, and commissural NTS subnuclei. Scale bars, 100 μm.
Extended Data Figure 7 Characterization of AdvillinCreERT2 and PvalbCre activity via Ai9 reporter.
a, tdTomato epifluorescence and DAPI staining in the lung from adult AdvillinCreERT2;Ai9+Tam reporter mice. TdTomato is not expressed in lung cells. Instead, tdTomato+ nerve fibres innervate the lung. b, tdTomato epifluorescence, CGRP and DAPI staining in lungs from adult AdvillinCreERT2;Ai9+Tam reporter mice. TdTomato is not expressed in NEBs. Dotted line demarcates the lung epithelium. Bronch, bronchioles. c, d, tdTomato epifluorescence in adult jugular–nodose complex (c) and tdTomato epifluorescence and DAPI staining in adult thoracic DRG (d) from AdvillinCreERT2;Ai9+Tam reporter mice. TdTomato is expressed in both the jugular–nodose complex (c) and the DRG (d). e, f, tdTomato epifluorescence and DAPI staining in the lung (e) and jugular–nodose complex (f) of adult PvalbCre;Ai9 reporter mice. TdTomato is not expressed in lung cells and neuronal cell bodies in the jugular–nodose complex. Scale bars, 100 μm.
Extended Data Figure 8 Respiratory properties of various Piezo2-deficient mouse lines.
a, b, Average frequency (a) and average tidal volume (b) of adult wild-type and Phox2bCre;Piezo2cKO mice under anaesthesia. *P < 0.05, unpaired Student’s t-test, mean ± s.e.m. c, Respiration activity during lung inflation (0.3 ml air) normalized to baseline in adult wild-type and AdvillinCreERT2;Piezo2cKO mice. *P < 0.05, unpaired Welch’s t-test, mean ± s.e.m. d, e, Phenylbiguanide (PBG)-induced chemoreflex in adult AdvillinCreERT2;Piezo2cKO mice. Representative traces of respiratory air flow from wild-type and AdvillinCreERT2;Piezo2cKO mice with 2.0 μg PBG intravenous injection (d). Baseline and longest breath interval after PBG injection. Bars represent mean ± s.e.m. (e). f, g, Average frequency (f) and average tidal volume (g) of adult wild-type and PvalbCre;Piezo2cKO mice under anaesthesia. Unpaired Student’s t-test, mean ± s.e.m. h, Respiration activity during lung inflation (0.3 ml air) normalized to baseline in adult wild-type and PvalbCre;Piezo2cKO mice. Unpaired Welch’s t-test, mean ± s.e.m. NS, statistically not significant.
Extended Data Figure 9 Roles of Piezo2 in respiratory system.
a, Piezo2 in nodose sensory neurons. It has been widely reported that nodose sensory neurons contain low-threshold mechanosensors innervating the lower airway tract including the lungs, while jugular sensory neurons contain high-threshold mechanosensors that innervate the upper airway tract, such as the larynx and the trachea. In addition, nodose sensory neurons project to the NTS6,53, a synaptic station required for the Hering–Breuer reflex in the brainstem54. Consistent with these findings, Piezo2 expression is detected in the jugular–nodose ganglia complex, and Piezo2+ nerve fibres project to the NTS. Moreover, adult mice lacking Piezo2 in the nodose ganglion show abolished vagal nerve responses to lung inflation, increased tidal volume, and an impaired Hering–Breuer inspiratory reflex. In addition to the nodose ganglia, Piezo2 is also expressed in the jugular, trigeminal, and dorsal root ganglia. We observed similar phenotypes in mice with Piezo2 depletion induced in virtually all sensory neurons in the adult. These data suggest that Piezo2 in nodose sensory neurons is the major stretch sensor required for lung volume regulation and the Hering–Breuer reflex response in adult mice. b, Piezo2 in jugular, trigeminal and/or spinal sensory neurons. In newborn mice, Piezo2 in sensory neurons of the neural crest origin is required for proper lung expansion and establishing efficient respiration as both global Piezo2 knockout and neural crest-derived sensory neuron-specific Piezo2 conditional knockout newborn mice showed hypoventilation, decreased inspiratory activity, altered expiratory pattern and unexpanded lungs. Our genetic studies also suggest that Piezo2 is not required in nodose ganglia for newborn lung expansion and respiration; however, this lack of requirement does not imply lack of involvement, and could be due to functional redundancy (that is, Piezo2 in jugular, trigeminal and/or dorsal root ganglia can compensate for Piezo2 deficiency in nodose ganglia). Although sensory neuronal control of respiration in newborn animals remains largely unknown, the newborn airway experiences a large pressure change in the course of lung expansion55. Piezo2-mediated mechanosensory feedback of the airway might be crucial for subsequent motor output (for example, control of diaphragm discharge or prevention of upper airway narrowing) to establish proper breathing patterns in newborn animals. The data presented here do not identify the exact cause of lethality in Piezo2-deficient newborn animals; however, we speculate that lethality in pups might be due to a combined effect of hypoventilation and lack of nutrients owing to inability to suckle. c, Piezo2 in NEBs. In addition to airway-innervating sensory neurons, Piezo2 is also expressed in pulmonary NEB cells, which are likely to be innervated by Piezo2+ afferents (Extended Data Fig. 3b, arrow). NEBs are enigmatic pulmonary cells whose physiological function is unclear3,22,23. Previous studies have suggested that the inflation-induced vagal nerve responses that are responsible for the Hering–Breuer reflex are slowly adapting2,4,5,7. While Piezo2 channels are generally rapidly adapting when assayed in cultured cells16, Piezo2 channels in Merkel cell–neurite complexes in the skin give rise to slowly adapting firing responses21,56 that are proposed to be caused by dual Piezo2 expression in both epidermal Merkel cells and associated afferents21,56. Therefore, it is possible that NEBs also contribute to sensing lung inflation in concert with Piezo2+ mechanosensory afferents. Future efforts will explore whether pulmonary NEBs function as mechanosensory cells, similar to Merkel cells.
Supplementary information
Respiratory distress observed in Piezo2-/- newborn mouse
Respiratory distress observed in Piezo2-/- newborn mouse. (MOV 1407 kb)
Respiratory distress observed in Wnt1Cre;Piezo2cKO newborn mouse
Respiratory distress observed in Wnt1Cre;Piezo2cKO newborn mouse. (MOV 2675 kb)
Rights and permissions
About this article
Cite this article
Nonomura, K., Woo, SH., Chang, R. et al. Piezo2 senses airway stretch and mediates lung inflation-induced apnoea. Nature 541, 176–181 (2017). https://doi.org/10.1038/nature20793
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nature20793
This article is cited by
-
The endothelium: gatekeeper to lung ischemia-reperfusion injury
Respiratory Research (2024)
-
Endophilin A2 controls touch and mechanical allodynia via kinesin-mediated Piezo2 trafficking
Military Medical Research (2024)
-
A vagal reflex evoked by airway closure
Nature (2024)
-
Latent neural population dynamics underlying breathing, opioid-induced respiratory depression and gasping
Nature Neuroscience (2024)
-
Probing corporeal awareness in women through virtual reality induction of embreathment illusion
Scientific Reports (2024)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.