Abstract
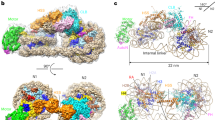
ISWI is a member of the SWI2/SNF2 family of chromatin remodellers1,2, which also includes Snf2, Chd1, and Ino80. ISWI is the catalytic subunit of several chromatin remodelling complexes, which mobilize nucleosomes along genomic DNA, promoting replication progression, transcription repression, heterochromatin formation, and many other nuclear processes3,4,5. The ATPase motor of ISWI is an autonomous remodelling machine6, whereas its carboxy (C)-terminal HAND–SAND–SLIDE (HSS) domain functions in binding extranucleosomal linker DNA7,8,9,10. The activity of the catalytic core of ISWI is inhibited by the regulatory AutoN and NegC domains, which are in turn antagonized by the H4 tail and extranucleosomal DNA, respectively, to ensure the appropriate chromatin landscape in cells11. How AutoN and NegC inhibit ISWI and regulate its nucleosome-centring activity remains elusive. Here we report the crystal structures of ISWI from the thermophilic yeast Myceliophthora thermophila and its complex with a histone H4 peptide. Our data show the amino (N)-terminal AutoN domain contains two inhibitory elements, which collectively bind the second RecA-like domain (core2), holding the enzyme in an inactive conformation. The H4 peptide binds to the core2 domain coincident with one of the AutoN-binding sites, explaining the ISWI activation by H4. The H4-binding surface is conserved in Snf2 and functions beyond AutoN regulation. The C-terminal NegC domain is involved in binding to the core2 domain and functions as an allosteric element for ISWI to respond to the extranucleosomal DNA length.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Manning, B. J. & Peterson, C. L. Releasing the brakes on a chromatin-remodeling enzyme. Nature Struct. Mol. Biol . 20, 5–7 (2013)
Clapier, C. R. & Cairns, B. R. The biology of chromatin remodeling complexes. Annu. Rev. Biochem. 78, 273–304 (2009)
Varga-Weisz, P. D. et al. Chromatin-remodelling factor CHRAC contains the ATPases ISWI and topoisomerase II. Nature 388, 598–602 (1997)
Tsukiyama, T., Daniel, C., Tamkun, J. & Wu, C. ISWI, a member of the SWI2/SNF2 ATPase family, encodes the 140 kDa subunit of the nucleosome remodeling factor. Cell 83, 1021–1026 (1995)
Ito, T., Bulger, M., Pazin, M. J., Kobayashi, R. & Kadonaga, J. T. ACF, an ISWI-containing and ATP-utilizing chromatin assembly and remodeling factor. Cell 90, 145–155 (1997)
Mueller-Planitz, F., Klinker, H., Ludwigsen, J. & Becker, P. B. The ATPase domain of ISWI is an autonomous nucleosome remodeling machine. Nature Struct. Mol. Biol . 20, 82–89 (2013)
Dang, W., Kagalwala, M. N. & Bartholomew, B. Regulation of ISW2 by concerted action of histone H4 tail and extranucleosomal DNA. Mol. Cell. Biol. 26, 7388–7396 (2006)
Grüne, T. et al. Crystal structure and functional analysis of a nucleosome recognition module of the remodeling factor ISWI. Mol. Cell 12, 449–460 (2003)
Hota, S. K. et al. Nucleosome mobilization by ISW2 requires the concerted action of the ATPase and SLIDE domains. Nature Struct. Mol. Biol . 20, 222–229 (2013)
Ludwigsen, J., Klinker, H. & Mueller-Planitz, F. No need for a power stroke in ISWI-mediated nucleosome sliding. EMBO Rep . 14, 1092–1097 (2013)
Clapier, C. R. & Cairns, B. R. Regulation of ISWI involves inhibitory modules antagonized by nucleosomal epitopes. Nature 492, 280–284 (2012)
Hauk, G., McKnight, J. N., Nodelman, I. M. & Bowman, G. D. The chromodomains of the Chd1 chromatin remodeler regulate DNA access to the ATPase motor. Mol. Cell 39, 711–723 (2010)
Xia, X., Liu, X., Li, T., Fang, X. & Chen, Z. Structure of chromatin remodeler Swi2/Snf2 in the resting state. Nature Struct. Mol. Biol . 23, 722–729 (2016)
Smith, C. L. & Peterson, C. L. A conserved Swi2/Snf2 ATPase motif couples ATP hydrolysis to chromatin remodeling. Mol. Cell. Biol. 25, 5880–5892 (2005)
Clapier, C. R. et al. Regulation of DNA translocation efficiency within the chromatin remodeler RSC/Sth1 potentiates nucleosome sliding and ejection. Mol. Cell 62, 453–461 (2016)
Dürr, H., Körner, C., Müller, M., Hickmann, V. & Hopfner, K. P. X-ray structures of the Sulfolobus solfataricus SWI2/SNF2 ATPase core and its complex with DNA. Cell 121, 363–373 (2005)
Dang, W. & Bartholomew, B. Domain architecture of the catalytic subunit in the ISW2-nucleosome complex. Mol. Cell. Biol. 27, 8306–8317 (2007)
Racki, L. R. et al. The histone H4 tail regulates the conformation of the ATP-binding pocket in the SNF2h chromatin remodeling enzyme. J. Mol. Biol. 426, 2034–2044 (2014)
Corona, D. F., Clapier, C. R., Becker, P. B. & Tamkun, J. W. Modulation of ISWI function by site-specific histone acetylation. EMBO Rep . 3, 242–247 (2002)
Clapier, C. R., Nightingale, K. P. & Becker, P. B. A critical epitope for substrate recognition by the nucleosome remodeling ATPase ISWI. Nucleic Acids Res . 30, 649–655 (2002)
Ferreira, H., Flaus, A. & Owen-Hughes, T. Histone modifications influence the action of Snf2 family remodelling enzymes by different mechanisms. J. Mol. Biol. 374, 563–579 (2007)
Deuring, R. et al. The ISWI chromatin-remodeling protein is required for gene expression and the maintenance of higher order chromatin structure in vivo . Mol. Cell 5, 355–365 (2000)
Saha, A., Wittmeyer, J. & Cairns, B. R. Chromatin remodeling through directional DNA translocation from an internal nucleosomal site. Nature Struct. Mol. Biol . 12, 747–755 (2005)
Zofall, M., Persinger, J., Kassabov, S. R. & Bartholomew, B. Chromatin remodeling by ISW2 and SWI/SNF requires DNA translocation inside the nucleosome. Nature Struct. Mol. Biol . 13, 339–346 (2006)
Leonard, J. D. & Narlikar, G. J. A nucleotide-driven switch regulates flanking DNA length sensing by a dimeric chromatin remodeler. Mol. Cell 57, 850–859 (2015)
Fairman-Williams, M. E., Guenther, U. P. & Jankowsky, E. SF1 and SF2 helicases: family matters. Curr. Opin. Struct. Biol. 20, 313–324 (2010)
Sen, P., Ghosh, S., Pugh, B. F. & Bartholomew, B. A new, highly conserved domain in Swi2/Snf2 is required for SWI/SNF remodeling. Nucleic Acids Res . 39, 9155–9166 (2011)
Sen, P. et al. The SnAC domain of SWI/SNF is a histone anchor required for remodeling. Mol. Cell. Biol. 33, 360–370 (2013)
Racki, L. R. et al. The chromatin remodeller ACF acts as a dimeric motor to space nucleosomes. Nature 462, 1016–1021 (2009)
Adams, P. D. et al. PHENIX: a comprehensive Python-based system for macromolecular structure solution. Acta Crystallogr. D 66, 213–221 (2010)
Yang, X., Zaurin, R., Beato, M. & Peterson, C. L. Swi3p controls SWI/SNF assembly and ATP-dependent H2A-H2B displacement. Nature Struct. Mol. Biol . 14, 540–547 (2007)
Acknowledgements
We thank S. Fan at the centre of structure biology (Tsinghua University) and the staff at beamline BL17U of Shanghai Synchrotron Radiation Facility for help with diffraction data collection, and the Tsinghua University Branch of the China National Center for Protein Sciences Beijing for providing facility support. This work was supported by the Chinese Key Research Plan-Protein Sciences (2014CB910100), the National Natural Science Foundation of China (31570731, 31270762), and the ‘Junior One Thousand Talents’ program to Z.C.
Author information
Authors and Affiliations
Contributions
L.Y. and L.W. prepared the proteins and performed the biochemical analyses with the help from X.X. and Y.T.; L.Y. crystallized the proteins; Z.C. wrote the manuscript with help from all authors; Z.C. directed and supervised all the research.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Additional information
Reviewer Information
Nature thanks B. Bartholomew and the other anonymous reviewer(s) for their contribution to the peer review of this work.
Extended data figures and tables
Extended Data Figure 1 Multiple sequence alignments of Chd1, Snf2, and ISWI subfamily of chromatin remodellers.
The sequence alignments were done with Clustal Omega. Secondary structural assignments on the top are based on the structure determined in this study and colour coded as in Fig. 1a. The residue numbering at the bottom is based on the sequence of MtISWI. The helicase motifs are assigned as reported13. The basic residues involved in AutoN inhibition are highlighted in magenta, and the acidic residues implicated in H4-binding are highlighted in yellow. The residues analysed in this study are indicated with red circles.
Extended Data Figure 2 Analyses of ISWI regulation by NegC.
a, Structure of the ISWI dimer. One molecule is coloured as in Fig. 1, and the other molecule is coloured grey. b, Chromatin remodelling of the 2D-V638D mutant (black). The activity of the parental 2D mutant was reproduced from Fig. 3e (red). Error bars, s.d. (n = 3). One representative gel was shown in the right panel. c, MALS of the core MtISWI (81–723; blue), 2D mutant (red), and mFL (81–1048; black). Core and mFL MtISWI are predominantly in a monomeric state, with a small fraction of dimer (~6%). The calculated molecular masses of the major peaks of core and mFL MtISWI are ~66 kDa and ~106 kDa, respectively, corresponding to a monomer, whereas the small peaks correspond to the dimer fractions. The 2D mutant shows two peaks with molecular masses of ~68 kDa and ~133 kDa, corresponding to a monomer and a dimer, respectively. d, Superimposition of the core2 domains of the two crystal structures examined in this study. For clarity, only the dimerization interface is shown. One dimer is coloured as in a; the other dimer is coloured yellow and blue. The core2 and NegC domains interact similarly in the two different crystal forms. The Brace helix shows some domain movement relative to NegC. e, ATPase activities of mFL, mFL-2D, and mFL-2D-II/DD in the absence (open bars) and the presence (filled bars) of DNA. Error bars, s.d. (n = 3). f, Representative gels of the overall chromatin remodelling assays of mFL, mFl-2D, and mFl-2D-II/DD. Quantifications of the cut fractions are shown in Fig. 4b.
Extended Data Figure 3 Structural comparisons among Snf2, Chd1, and ISWI.
a, b, Comparisons of the overall structures of MtISWI and MtSnf2 (Protein Data Bank accession number 5HZR)13. The structures of the core2 domains are aligned. The core1 domains are shown as surface presentations, which orient differently in these two proteins. ISWI is coloured as in Fig. 1. The core1 and SnAc domains of MtSnf2 are coloured grey and blue, respectively. Motif V (R567 of MtISWI and R950 of MtSnf2) and the acidic patch of the core2 domain implicated in H4-binding are coloured gold. c, d, Comparisons of the overall structures of MtISWI and ScChd1 (Protein Data Bank accession number 3MWY)12. The structures of the core2 domains are aligned. The core1 domains are shown as surface presentations. The N-terminal dCD and the C-terminal bridge of ScChd1 are coloured pink and red, respectively. The NegC domain of ISWI extends outwards, whereas the C-terminal bridge of Chd1 binds to the core1 domain intramolecularly. e, Structural alignment of the core1 domains of MtISWI (green), MtSnf2 (blue), and ScChd1 (grey). f, Structural alignment of the core2 domains of MtISWI (cyan), MtSnf2 (blue), and ScChd1 (grey). The DNA-binding elements identified in MtSnf2 (K662 and R950) and ScChd1 (R750 and R772) are conserved among these remodellers, whereas K692 of MtSnf2 is unique to the Snf2-subfamily proteins. The arginine-fingers of MtISWI (R599 and R602), MtSnf2 (R982 and R985), and ScChd1 (R804 and R807) are conserved (Extended Data Fig. 1). The Brace helices of the remodellers show different lengths. g, Comparisons of lobe1 of MtISWI, MtSnf2 and ScChd1. The structures of the core1 domains are aligned. The N-terminal auxiliary domains of MtISWI (AutoN), MtSnf2 (postHSA), and ScChd1 (dCD) interact with the core1 domain differently. h, Comparisons of lobe2 of MtISWI, MtSnf2, and ScChd1. The structures of the core2 domains are aligned. The C-terminal auxiliary domains of MtISWI (NegC), MtSnf2 (SnAC), and ScChd1 (bridge) interact with the core2 domain differently.
Extended Data Figure 4 Analyses of ISWI regulation by AutoN.
a, Representative curves of the MESG-based assays to measure the ATPase activities of MtISWI (81–723) with intact interface (Core; black), R149A/R151A (2RA; blue), and R141A/R149A/R151A (3RA; red). The assays were performed in the absence (open circle) and presence (filled circle) of DNA. The rates of ATP hydrolysis were extracted from the slops of the curves in the linear ranges. The activities were normalized to the ATPase activity of the Core protein in the presence of DNA. The right panel shows the quantification of the measurements in the presence (filled bars) and absence (open bars) of DNA. Error bars, s.d. (n = 3). b, Gels of the restriction-enzyme-accessibility assays of MtISWI (81–723) with the intact interface (Core) and five L3 loop mutants. The assays were performed with 3 nM Cy5-labelled mononucleosomes, and 5 μM of various ISWI proteins at the indicated time points. Owing to the very low activity of the enzymes, a large excess of the proteins was used. c, Quantification of the remodelling activities in b. Core, black; R151A, green; R149A, cyan; R141A, brown; 2RA, blue; 3RA, red. Error bars, s.d. (n = 3). d, Gels of the restriction-enzyme-accessibility assays of MtISWI (81–723) with the intact interface (Core), ΔL3 loop, and Δα4 mutants. Quantification of the remodelling activities is showed in Fig. 2f. The assays were performed with 3 nM mononucleosomes and 0.2 μM of various ISWI proteins. e, Gels of the restriction-enzyme-accessibility assays of core2 mutants of MtISWI (81–723). The cut fractions were quantified and shown in Fig. 2i. Three independent assays were performed and one is shown.
Extended Data Figure 5 Analyses of the interaction between the H4 tail peptide and ISWI.
a, Superimposition of the final structure around the H4 peptide (yellow) with the omit difference map (grey, Fo − Fc, contour level σ = 2) before the H4 peptide was modelled into the structure. b–e, ITC analyses of the interactions between the core2 domain of MtISWI and various H4 peptides. b, Wild-type unmodified H4 peptide; c, R17A mutant H4 peptide; d, acetylated H4K16 peptide; e, R19A mutant H4 peptide.
Extended Data Figure 6 Analyses of the H4-binding surface of ISWI.
a, GST pull-down assays. GST–H4 pulled down a significant amount of the 2D mutant MtISWI (81–723) (lane 4). Introduction of additional D520A mutation showed a mild defect in H4-binding (lane 7). The mutations of D524A and E474A dramatically reduced the binding (lanes 10 and 13, respectively). b, Gels of the restriction-enzyme-accessibility assays of the H4-binding interface mutants. The assays were performed with 3 nM Cy5-labelled mononucleosomes and 0.2 μM ISWI proteins. The cut fractions were quantified and shown in Fig. 3e. c, ATPase activities of MtISWI (81–723) with the intact interface (Core) and two mutants of the H4-binding surface (E474A and D524A) in the absence (open bars) and the presence (filled bars) of DNA. Error bars, s.d. (n = 3). d, Chromatin remodelling activities of Core (black), E474A (blue) and D524A (red). The bottom panels show the representative gels of the chromatin remodelling assays. The assays were performed with 3 nM Cy5-labelled mononucleosomes and 5 μM ISWI proteins. Error bars, s.d. (n = 3).
Supplementary information
Supplementary Figure
This file contains Supplementary Figure 1, gel source data. (PDF 1370 kb)
Rights and permissions
About this article
Cite this article
Yan, L., Wang, L., Tian, Y. et al. Structure and regulation of the chromatin remodeller ISWI. Nature 540, 466–469 (2016). https://doi.org/10.1038/nature20590
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nature20590
This article is cited by
-
Structure of the ISW1a complex bound to the dinucleosome
Nature Structural & Molecular Biology (2024)
-
Energy-driven genome regulation by ATP-dependent chromatin remodellers
Nature Reviews Molecular Cell Biology (2023)
-
The role of auxiliary domains in modulating CHD4 activity suggests mechanistic commonality between enzyme families
Nature Communications (2022)
-
The emerging role of ISWI chromatin remodeling complexes in cancer
Journal of Experimental & Clinical Cancer Research (2021)
-
The limits of clinical findings in similar phenotypes, from Carpenter to ATRX syndrome using a whole exome sequencing approach: a case review
Human Genomics (2021)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.