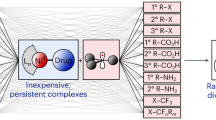
Abstract
Owing to the limited availability of natural sources, the widespread demand of the flavouring, perfume and pharmaceutical industries for unsaturated alcohols is met by producing them from α,β-unsaturated aldehydes, through the selective hydrogenation of the carbon–oxygen group (in preference to the carbon–carbon group)1. However, developing effective catalysts for this transformation is challenging2,3,4,5,6,7, because hydrogenation of the carbon–carbon group is thermodynamically favoured8. This difficulty is particularly relevant for one major category of heterogeneous catalyst: metal nanoparticles supported on metal oxides. These systems are generally incapable of significantly enhancing the selectivity towards thermodynamically unfavoured reactions, because only the edges of nanoparticles that are in direct contact with the metal-oxide support possess selective catalytic properties; most of the exposed nanoparticle surfaces do not9,10,11,12,13,14. This has inspired the use of metal–organic frameworks (MOFs) to encapsulate metal nanoparticles within their layers or inside their channels, to influence the activity of the entire nanoparticle surface while maintaining efficient reactant and product transport owing to the porous nature of the material15,16,17,18. Here we show that MOFs can also serve as effective selectivity regulators for the hydrogenation of α,β-unsaturated aldehydes. Sandwiching platinum nanoparticles between an inner core and an outer shell composed of an MOF with metal nodes of Fe3+, Cr3+ or both (known as MIL-101; refs 19, 20, 21) results in stable catalysts that convert a range of α,β-unsaturated aldehydes with high efficiency and with significantly enhanced selectivity towards unsaturated alcohols. Calculations reveal that preferential interaction of MOF metal sites with the carbon–oxygen rather than the carbon–carbon group renders hydrogenation of the former by the embedded platinum nanoparticles a thermodynamically favoured reaction. We anticipate that our basic design strategy will allow the development of other selective heterogeneous catalysts for important yet challenging transformations.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
Change history
02 October 2016
A footnote symbol was corrected in Table 1
References
Gallezot, P. & Richard, D. Selective hydrogenation of α,β-unsaturated aldehydes. Catal. Rev. Sci. Eng. 40, 81–126 (1998)
Tian, Z., Xiang, X., Xie, L. & Li, F. Liquid-phase hydrogenation of cinnamaldehyde: enhancing selectivity of supported gold catalysts by incorporation of cerium into the support. Ind. Eng. Chem. Res. 52, 288–296 (2013)
Kahsar, K. R., Schwartz, D. K. & Medlin, J. W. Control of metal catalyst selectivity through specific noncovalent molecular interactions. J. Am. Chem. Soc. 136, 520–526 (2014)
Wu, B., Huang, H., Yang, J., Zheng, N. & Fu, G. Selective hydrogenation of α,β-unsaturated aldehydes catalyzed by amine-capped platinum-cobalt nanocrystals. Angew. Chem. Int. Edn 51, 3440–3443 (2012)
Stassi, J. P., Zgolicz, P. D., de Miguel, S. R. & Scelza, O. A. Formation of different promoted metallic phases in PtFe and PtSn catalysts supported on carbonaceous materials used for selective hydrogenation. J. Catal. 306, 11–29 (2013)
Kennedy, G., Baker, L. R. & Somorjai, G. A. Selective amplification of C=O bond hydrogenation on Pt/TiO2: catalytic reaction and sum-frequency generation vibrational spectroscopy studies of crotonaldehyde hydrogenation. Angew. Chem. Int. Edn 53, 3405–3408 (2014)
Kliewer, C. J., Bieri, M. & Somorjai, G. A. Hydrogenation of the α,β-unsaturated aldehydes acrolein, crotonaldehyde, and prenal over Pt single crystals: a kinetic and sum-frequency generation vibrational spectroscopy study. J. Am. Chem. Soc. 131, 9958–9966 (2009)
Ide, M. S., Hao, B., Neurock, M. & Davis, R. J. Mechanistic insights on the hydrogenation of α,β-unsaturated ketones and aldehydes to unsaturated alcohols over metal catalysts. ACS Catal. 2, 671–683 (2012)
Cargnello, M. et al. Control of metal nanocrystal size reveals metal-support interface role for ceria catalysts. Science 341, 771–773 (2013)
Corma, A. & Serna, P. Chemoselective hydrogenation of nitro compounds with supported gold catalysts. Science 313, 332–334 (2006)
Ma, C. Y. et al. Mesoporous Co3O4 and Au/Co3O4 catalysts for low-temperature oxidation of trace ethylene. J. Am. Chem. Soc. 132, 2608–2613 (2010)
Fu, Q. et al. Interface-confined ferrous centers for catalytic oxidation. Science 328, 1141–1144 (2010)
Vayssilov, G. N. et al. Support nanostructure boosts oxygen transfer to catalytically active platinum nanoparticles. Nature Mater . 10, 310–315 (2011)
Ghosh, S. et al. Selective oxidation of propylene to propylene oxide over silver-supported tungsten oxide nanostructure with molecular oxygen. ACS Catal. 4, 2169–2174 (2014)
Furukawa, H., Cordova, K. E., O’Keeffe, M. & Yaghi, O. M. The chemistry and applications of metal-organic frameworks. Science 341, 1230444 (2013)
McDonald, T. M. et al. Cooperative insertion of CO2 in diamine-appended metal-organic frameworks. Nature 519, 303–308 (2015)
Dhakshinamoorthy, A. & Garcia, H. Catalysis by metal nanoparticles embedded on metal-organic frameworks. Chem. Soc. Rev. 41, 5262–5284 (2012)
Lu, G. et al. Imparting functionality to a metal-organic framework material by controlled nanoparticle encapsulation. Nature Chem . 4, 310–316 (2012)
Férey, G. et al. A chromium terephthalate-based solid with unusually large pore volumes and surface area. Science 309, 2040–2042 (2005)
Taylor-Pashow, K. M. L., Rocca, J. D., Xie, Z., Tran, S. & Lin, W. Postsynthetic modifications of iron-carboxylate nanoscale metal-organic frameworks for imaging and drug delivery. J. Am. Chem. Soc. 131, 14261–14263 (2009)
Bauer, S. et al. High-throughput assisted rationalization of the formation of metal organic frameworks in the iron (III) aminoterephthalate solvothermal system. Inorg. Chem. 47, 7568–7576 (2008)
Xiao, D. J. et al. Oxidation of ethane to ethanol by N2O in a metal-organic framework with coordinatively unsaturated iron (II) sites. Nature Chem . 6, 590–595 (2014)
Hu, P., Morabito, J. V. & Tsung, C.-K. Core-shell catalysts of metal nanoparticle core and metal-organic framework shell. ACS Catal. 4, 4409–4419 (2014)
Guo, Z. et al. Carbon nanotube-supported Pt-based bimetallic catalysts prepared by a microwave-assisted polyol reduction method and their catalytic applications in the selective hydrogenation. J. Catal. 276, 314–326 (2010)
Nørskov, J. K. et al. Trends in the exchange current for hydrogen evolution. J. Electrochem. Soc. 152, J23–J26 (2005)
Zhu, Y., Qian, H., Drake, B. A. & Jin, R. Atomically precise Au25(SR)18 nanoparticles as catalysts for the selective hydrogenation of α,β-unsaturated ketones and aldehydes. Angew. Chem. Int. Edn 49, 1295–1298 (2010)
Cano, I., Chapman, A. M., Urakawa, A. & van Leeuwen, P. W. N. M. Air-stable gold nanoparticles ligated by secondary phosphine oxides for the chemoselective hydrogenation of aldehydes: crucial role of the ligand. J. Am. Chem. Soc. 136, 2520–2528 (2014)
Morris, W. et al. Synthesis, structure, and metalation of two new highly porous zirconium metal−organic frameworks. Inorg. Chem. 51, 6443–6445 (2012)
Glover, T. G., Peterson, G. W., Schindler, B. J., Britt, D. & Yaghi, O. M. MOF-74 building unit has a direct impact on toxic gas adsorption. Chem. Eng. Sci. 66, 163–170 (2011)
Na, K., Choi, K. M., Yaghi, O. M. & Somorjai, G. A. Metal nanocrystals embedded in single nanocrystals of MOFs give unusual selectivity as heterogeneous catalysts. Nano Lett. 14, 5979–5983 (2014)
Acknowledgements
This work was supported financially by the National Research Fund for Fundamental Key Project (grant 2014CB931801 to Z.T.), the National Natural Science Foundation of China (grants 21475029 and 91427302 to Z.T., and 21303029 to G.L.), the Instrument Developing Project of the Chinese Academy of Sciences (grant YZ201311 to Z.T.), the CAS-CSIRO Cooperative Research Program (grant GJHZ1503 to Z.T.), the Strategic Priority Research Program of the Chinese Academy of Sciences (grant XDA09040100 to Z.T.), and the Youth Innovation Promotion Association CAS (grant 2016036 to G.L.). We gratefully acknowledge the use of the supercomputer facilities at the National Computational Infrastructure (NCI) in Canberra, Australia.
Author information
Authors and Affiliations
Contributions
Z.T. and G.L. proposed the research direction and guided the project. M.Z., K.Y. and G.L. designed and performed the materials synthesis, characterization, and catalytic tests. Y.W. and H.Z. performed the theoretical calculations and explained the catalytic results. J.G. helped to detect and analyse the Brunauer–Emmett–Teller (BET) surface areas and pore-size distributions of samples. L.G. guided the high-angle annular dark-field scanning transmission electron microscopy (HAADF-STEM) imaging of sandwich structures. W.H. took part in the characterization of some samples, and discussed the results. Z.T., G.L., M.Z. and Y.W. drafted the manuscript. All of the authors discussed the results and commented on the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Additional information
Reviewer Information Nature thanks P. Claus and the other anonymous reviewer(s) for their contribution to the peer review of this work.
Supplementary information
Supplementary Information
This file contains Supplementary Methods, Supplementary Text, Supplementary Notes 1-5, Supplementary Figures 1-54, Supplementary Tables 1-11 and Supplementary references. (PDF 14827 kb)
Rights and permissions
About this article
Cite this article
Zhao, M., Yuan, K., Wang, Y. et al. Metal–organic frameworks as selectivity regulators for hydrogenation reactions. Nature 539, 76–80 (2016). https://doi.org/10.1038/nature19763
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nature19763
This article is cited by
-
Spectroscopic visualization of reversible hydrogen spillover between palladium and metal–organic frameworks toward catalytic semihydrogenation
Nature Communications (2024)
-
Self-assembled hydrated copper coordination compounds as ionic conductors for room temperature solid-state batteries
Nature Communications (2024)
-
Recent progress on the synthesis of defective UiO-66 for thermal catalysis
Nano Research (2024)
-
Incorporating metal nanoparticles in porous materials via selective heating effect using microwave
Nano Research (2024)
-
Nitro-functionalized Fe-MOFs for lithium-sulfur batteries
Ionics (2024)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.