Abstract
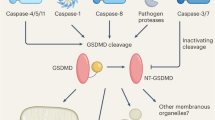
Inflammatory caspases cleave the gasdermin D (GSDMD) protein to trigger pyroptosis, a lytic form of cell death that is crucial for immune defences and diseases. GSDMD contains a functionally important gasdermin-N domain that is shared in the gasdermin family. The functional mechanism of action of gasdermin proteins is unknown. Here we show that the gasdermin-N domains of the gasdermin proteins GSDMD, GSDMA3 and GSDMA can bind membrane lipids, phosphoinositides and cardiolipin, and exhibit membrane-disrupting cytotoxicity in mammalian cells and artificially transformed bacteria. Gasdermin-N moved to the plasma membrane during pyroptosis. Purified gasdermin-N efficiently lysed phosphoinositide/cardiolipin-containing liposomes and formed pores on membranes made of artificial or natural phospholipid mixtures. Most gasdermin pores had an inner diameter of 10–14 nm and contained 16 symmetric protomers. The crystal structure of GSDMA3 showed an autoinhibited two-domain architecture that is conserved in the gasdermin family. Structure-guided mutagenesis demonstrated that the liposome-leakage and pore-forming activities of the gasdermin-N domain are required for pyroptosis. These findings reveal the mechanism for pyroptosis and provide insights into the roles of the gasdermin family in necrosis, immunity and diseases.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
Change history
10 June 2016
An error in the Reviewer Information section was corrected.
05 October 2016
An Erratum to this paper has been published: https://doi.org/10.1038/nature20106
References
Jorgensen, I. & Miao, E. A. Pyroptotic cell death defends against intracellular pathogens. Immunol. Rev. 265, 130–142 (2015)
Cookson, B. T. & Brennan, M. A. Pro-inflammatory programmed cell death. Trends Microbiol. 9, 113–114 (2001)
Shi, J. et al. Inflammatory caspases are innate immune receptors for intracellular LPS. Nature 514, 187–192 (2014)
Kayagaki, N. et al. Non-canonical inflammasome activation targets caspase-11. Nature 479, 117–121 (2011)
Lamkanfi, M. & Dixit, V. M. Mechanisms and functions of inflammasomes. Cell 157, 1013–1022 (2014)
Zhao, Y. & Shao, F. Diverse mechanisms for inflammasome sensing of cytosolic bacteria and bacterial virulence. Curr. Opin. Microbiol. 29, 37–42 (2016)
Hagar, J. A., Powell, D. A., Aachoui, Y., Ernst, R. K. & Miao, E. A. Cytoplasmic LPS activates caspase-11: implications in TLR4-independent endotoxic shock. Science 341, 1250–1253 (2013)
Kayagaki, N. et al. Noncanonical inflammasome activation by intracellular LPS independent of TLR4. Science 341, 1246–1249 (2013)
Yang, J., Zhao, Y. & Shao, F. Non-canonical activation of inflammatory caspases by cytosolic LPS in innate immunity. Curr. Opin. Immunol. 32, 78–83 (2015)
Shi, J. et al. Cleavage of GSDMD by inflammatory caspases determines pyroptotic cell death. Nature 526, 660–665 (2015)
Kayagaki, N. et al. Caspase-11 cleaves gasdermin D for non-canonical inflammasome signalling. Nature 526, 666–671 (2015)
Saeki, N. & Sasaki, H. in Endothelium and Epithelium: Composition, Functions, and Pathology (eds Carrasco, J. & Matheus, M. ) Ch. IX, 193–211 (Nova Science Publishers, 2011)
Tanaka, S., Mizushina, Y., Kato, Y., Tamura, M. & Shiroishi, T. Functional conservation of Gsdma cluster genes specifically duplicated in the mouse genome. G3 (Bethesda) 3, 1843–1850 (2013)
Sato, H. et al. A new mutation Rim3 resembling Reden is mapped close to retinoic acid receptor alpha (Rara) gene on mouse chromosome 11. Mamm. Genome 9, 20–25 (1998)
Porter, R. M. et al. Defolliculated (dfl): a dominant mouse mutation leading to poor sebaceous gland differentiation and total elimination of pelage follicles. J. Invest. Dermatol. 119, 32–37 (2002)
Runkel, F. et al. The dominant alopecia phenotypes Bareskin, Rex-denuded, and Reduced Coat 2 are caused by mutations in gasdermin 3. Genomics 84, 824–835 (2004)
Van Laer, L. et al. Nonsyndromic hearing impairment is associated with a mutation in DFNA5. Nat. Genet. 20, 194–197 (1998)
Delmaghani, S. et al. Mutations in the gene encoding pejvakin, a newly identified protein of the afferent auditory pathway, cause DFNB59 auditory neuropathy. Nat. Genet. 38, 770–778 (2006)
Shepard, L. A., Shatursky, O., Johnson, A. E. & Tweten, R. K. The mechanism of pore assembly for a cholesterol-dependent cytolysin: formation of a large prepore complex precedes the insertion of the transmembrane beta-hairpins. Biochemistry 39, 10284–10293 (2000)
Fink, S. L., Bergsbaken, T. & Cookson, B. T. Anthrax lethal toxin and Salmonella elicit the common cell death pathway of caspase-1-dependent pyroptosis via distinct mechanisms. Proc. Natl Acad. Sci. USA 105, 4312–4317 (2008)
Holm, L. & Rosenström, P. Dali server: conservation mapping in 3D. Nucleic Acids Res. 38, W545–W549 (2010)
Delmaghani, S. et al. Hypervulnerability to sound exposure through impaired adaptive proliferation of peroxisomes. Cell 163, 894–906 (2015)
Van Rossom, S., Op de Beeck, K., Hristovska, V., Winderickx, J. & Van Camp, G. The deafness gene DFNA5 induces programmed cell death through mitochondria and MAPK-related pathways. Front. Cell. Neurosci. 9, 231 (2015)
Bischofberger, M., Iacovache, I. & van der Goot, F. G. Pathogenic pore-forming proteins: function and host response. Cell Host Microbe 12, 266–275 (2012)
Hildebrand, J. M. et al. Activation of the pseudokinase MLKL unleashes the four-helix bundle domain to induce membrane localization and necroptotic cell death. Proc. Natl Acad. Sci. USA 111, 15072–15077 (2014)
Dondelinger, Y. et al. MLKL compromises plasma membrane integrity by binding to phosphatidylinositol phosphates. Cell Reports 7, 971–981 (2014)
Wang, H. et al. Mixed lineage kinase domain-like protein MLKL causes necrotic membrane disruption upon phosphorylation by RIP3. Mol. Cell 54, 133–146 (2014)
Cai, Z. et al. Plasma membrane translocation of trimerized MLKL protein is required for TNF-induced necroptosis. Nat. Cell Biol. 16, 55–65 (2014)
Iacovache, I., Bischofberger, M. & van der Goot, F. G. Structure and assembly of pore-forming proteins. Curr. Opin. Struct. Biol. 20, 241–246 (2010)
Reboul, C. F., Whisstock, J. C. & Dunstone, M. A. Giant MACPF/CDC pore forming toxins: A class of their own. Biochim. Biophys. Acta 1858, 475–486 (2016)
Zhao, Y. et al. The NLRC4 inflammasome receptors for bacterial flagellin and type III secretion apparatus. Nature 477, 596–600 (2011)
Gong, Y.-N. et al. Chemical probing reveals insights into the signaling mechanism of inflammasome activation. Cell Res. 20, 1289–1305 (2010)
Wilschut, J. & Papahadjopoulos, D. Ca2+-induced fusion of phospholipid vesicles monitored by mixing of aqueous contents. Nature 281, 690–692 (1979)
Tang, G. et al. EMAN2: an extensible image processing suite for electron microscopy. J. Struct. Biol. 157, 38–46 (2007)
Scheres, S. H. A Bayesian view on cryo-EM structure determination. J. Mol. Biol. 415, 406–418 (2012)
Frank, J. et al. SPIDER and WEB: processing and visualization of images in 3D electron microscopy and related fields. J. Struct. Biol. 116, 190–199 (1996)
Otwinowski, Z. & Minor, W. in Methods in Enzymology Vol. 276 (eds Carter, C. W. Jr & Sweet, R. M. ) 307–326 (Academic, 1997)
Adams, P. D. et al. PHENIX: a comprehensive Python-based system for macromolecular structure solution. Acta Crystallogr. D 66, 213–221 (2010)
Emsley, P., Lohkamp, B., Scott, W. G. & Cowtan, K. Features and development of Coot. Acta Crystallogr. D 66, 486–501 (2010)
Chen, V. B. et al. MolProbity: all-atom structure validation for macromolecular crystallography. Acta Crystallogr. D 66, 12–21 (2010)
Eswar, N. et al. Comparative protein structure modeling using MODELLER. Curr. Protoc. Protein Sci. Chapter 2, Unit 2.9 (2007)
Shen, M. Y. & Sali, A. Statistical potential for assessment and prediction of protein structures. Protein Sci. 15, 2507–2524 (2006)
Acknowledgements
We thank W. Wei for reagents, H. Wang for suggestions on electron microscopy data analysis, and the staff of beamlines BL18U1 and BL19U1 at National Center for Protein Sciences, Shanghai, and Shanghai Synchrotron Radiation Facility for X-ray data collection. This work was supported by grants from the Strategic Priority Research Program of the Chinese Academy of Sciences (XDB08020202), the China National Science Foundation Program for Distinguished Young Scholars (31225002) and Program for International Collaborations (31461143006), and the National Basic Research Program of China 973 Program (2012CB518700 and 2014CB849602) to F.S. The research was also supported in part by an International Early Career Scientist grant from the Howard Hughes Medical Institute and the Beijing Scholar Program to F.S.
Author information
Authors and Affiliations
Contributions
J.D., D.-C.W. and F.S. conceived the study; J.D., together with K.W., designed and performed the majority of the experiments; Y.S. helped with protein purification; W.L. performed the pyroptosis assay; Q.S. assisted J.D. in electron microscopy studies; J.S. provided critical reagents and suggestions; H.S. performed structural modelling; and J.D. and F.S. analysed the data and wrote the manuscript. All authors discussed the results and commented on the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Additional information
Reviewer Information Nature thanks F. Sigworth and the other anonymous reviewer(s) for their contribution to the peer review of this work.
Extended data figures and tables
Extended Data Figure 1 Multiple gasdermin-N domains can induce mammalian cell pyroptosis and also exhibit cytotoxicity in bacteria.
a, b, Full-length (FL) or N-terminal domain regions of different gasdermin-family members were transfected into 293T cells for 20 h. Human GSDMD and mouse GSDMA3 had an N-terminal 3 × Flag tag and human GSDMA, GSDMB, GSDMC and DFNA5 had a C-terminal Flag tag. ATP-based cell viability is expressed as mean ± s.d. from three technical replicates (a). Representative views of cell death morphology are shown in b. c, d, Cytotoxicity of the gasdermin-N domain in bacteria. Indicated gasdermins were cloned into an IPTG-inducible vector for transformation into E. coli. c, Representative agar plates showing transformed E. coli colonies for GSDMD. d, Bacterial colony-forming units (CFU) per transformation for GSDMD and other gasdermins are shown in the logarithmic form (log10) as mean ± s.d. from three technical replicates. All data shown are representative of three independent experiments.
Extended Data Figure 2 Membrane phospholipid binding of the gasdermin-N domain.
a–d, f, Liposomes with indicated lipid compositions (a–d) or prepared using bovine liver or brain-derived polar lipid extracts (f) were incubated with purified gasdermin proteins. After ultracentrifugation, the liposome-free supernatant (S) and liposome pellet (P) were analysed by SDS–PAGE and Coomassie blue staining. e, Noncovalent complex of cleaved GSDMD and GSDMA3 with a Flag tag attached to the end of the gasdermin-N domain or the corresponding uncleaved full-length proteins were incubated with the lipid strips, and the strips were then probed with the anti-Flag antibody. Right, protein loading control. All data shown are representative of three independent experiments.
Extended Data Figure 3 Biomembrane association and lysis by the gasdermin-N domain.
a, b, Subcellular fractionation of the gasdermin-N domains of GSDMD and GSDMA during pyroptosis. Gsdmd−/− iBMDMs expressing 2 × Flag and haemagglutinin (HA)-tagged GSDMD were untreated or stimulated with LPS electroporation (a). 293T cells expressing PPase-cleavable Flag–GSDMA were untreated or electroporated with purified PPase (b). Homogenized cell extracts were sequentially centrifuged at 700g, 20,000g and 100,000g to separate membrane fractions (P7, P20 and P100) from the S100 soluble fraction. The factions were immunoblotted as indicated. c, Microscopy of GSDMA3-N domain localization in cells undergoing pyroptosis. The gasdermin-N domain of GSDMA3 (GSDMA3-N(L184D)) fused N-terminally to eGFP was stably expressed in HeLa cells under a tetracycline-inducible promoter. Shown are representative time-lapse cell images (brightfield and fluorescence) taken from 4–5 h after doxycycline addition. Scale bar, 15 μm. For videos of two representative cells, see Supplementary Videos 3 and 4. d, e, Effects of extracellular or intracellular delivery of purified gasdermin proteins on 293T cell viability. Equal amounts of indicated gasdermin proteins or PFO were added directly into cell culture medium (d) or electroporated into the cytosol (e). ATP-based cell viability is expressed as mean ± s.d. from three technical replicates. f, g, Bacterial protoplast lysis by purified gasdermin proteins. Protoplasts of B. megaterium were treated with indicated gasdermin proteins or PFO. Membrane lysis was assessed by measuring the OD600 of the protoplasts. Triton-X 100 treatment was used to achieve 100% lysis of the protoplasts. Time-course measurement of GSDMD treatment is shown in f. Relative protoplast lysis by GSDMD and other gasdermins is expressed as mean ± s.d. from three technical replicates (g). All data shown are representative of three independent experiments.
Extended Data Figure 4 Liposome-leakage-inducing activity of the gasdermin-N domain.
a–c, Liposomes with indicated lipid compositions were treated with purified gasdermin proteins or PFO as indicated. Liposome leakage was monitored by measuring DPA chelating-induced fluorescence of released Tb3+. Time course of relative Tb3+ release is shown. A dose titration of GSDMA proteins is shown in b. CTL, control. All data shown are representative of three independent experiments.
Extended Data Figure 5 Membrane binding-induced oligomerization of and pore formation by the gasdermin-N domain.
a, Gel filtration chromatography of full-length GSDMD, GSDMA and GSDMA3. Indicated gasdermin proteins were loaded on the Superdex G75 column. Arrows indicate elution volume of the molecular mass markers. b, Oligomerization of gasdermin-N domain on the liposome membrane. Indicated gasdermin proteins or PFO were incubated with cardiolipin or cholesterol liposomes, respectively. Intact proteins or proteins associated with the liposomes were mock treated or treated with glutaraldehyde and analysed by SDS-agarose gel electrophoresis and Coomassie blue staining. The gasdermin-C domain migrating at the bottom of the gel was omitted for clarity. c, Oligomerization of the gasdermin-N domain during pyroptosis. To trigger pyroptosis, Gsdmd−/− iBMDMs expressing 2 × Flag-HA–GSDMD and HeLa cells expressing the PPase-cleavable Flag–GSDMA were electroporated with LPS and PPase, respectively. The cytosol (S) and membrane (P) fractions from unstimulated and pyroptotic cells were subjected to glutaraldehyde-mediated crosslinking followed by SDS-agarose (top) or SDS–PAGE (bottom) gel electrophoresis. d, Pore-forming activity of the gasdermin-N domain. Liposomes with 80% phosphatidylcholine and 20% PtdIns(4, 5)P2 were treated with indicated gasdermin proteins. Shown are representative negative-stain electron microscopy micrographs of the liposomes (scale bar, 100 nm). All data shown are representative of three independent experiments.
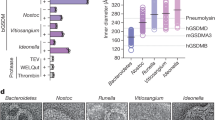
Extended Data Figure 6 Analyses of the gasdermin pore.
a, b, Size distribution of GSDMD and GSDMA3 pores formed on cardiolipin liposomes. The inner diameters of pores were measured and plotted. A total of 200 or 100 pores for 5 μM or 0.5 μM GSDMD/GSDMA3-treated liposome samples, respectively, were randomly selected from the negative-stain electron microscopy micrographs in Fig. 3a. c, Effects of different PEG molecules on lactate dehydrogenase (LDH) release from caspase-1-mediated pyroptotic cells. iBMDMs harbouring a sensitive Nlrp1b allele were treated with indicated mass concentration of different PEG molecules and then stimulated with anthrax lethal toxin or LFn-BsaK to activate the canonical NLRP1B or NAIP2/NLRC4 inflammasomes, respectively. 1.2% PEG200 and 12% PEG2000 (mass concentration) have roughly the same molar concentration. Shown are LDH release expressed as mean ± s.d. from three technical replicates. d, Pores formed by active GSDMD and GSDMA3 on monolayer membranes containing 80% phosphatidylcholine and 20% cardiolipin. Shown are representative negative-stain electron microscopy micrograph images (scale bar, 100 nm). e, Symmetry determination of the gasdermin pore. GSDMA3 pores formed on the monolayer membrane (d) were subjected to 2D reference-free classification. One class of pores with the best particle contrast were subjected to rotational auto-correlation calculation and the inlet electron microscopy image (scale bar, 20 nm) shows the averaged view of the class of pores (242 particles). The analyses revealed 16-fold symmetry. Data shown in a–d are representative of three independent experiments.
Extended Data Figure 7 Crystal structure of GSDMA3 and Dali search results for its gasdermin-N domain.
a, 2Fo − Fc electron density map (contoured at 1.0σ) of GSDMA3 gasdermin-N domain (GSDMA3-N) structure. b, Cartoon diagram of GSDMA3-N structure. c, Structural model of GSDMD obtained from homology modelling and the conserved autoinhibitory interactions. Bottom, overall structure of modelled GSDMD; top, comparisons of the hydrophobic core (left) and the second inter-domain contact (right) with the corresponding structures in GSDMA3. Conserved residues involved in the autoinhibitory interactions are labelled and shown as sticks. Cyan, GSDMD-N; orange, GSDMD-C; green, GSDMA3-N; yellow, GSDMA3-C. d, Dali search results for the GSDMA3-N structure.
Extended Data Figure 8 Multiple sequence alignment of gasdermin family members.
GSDMA3 is a mouse protein and sequences of other gasdermins are from human. The secondary structures determined from GSDMA3 are marked along the sequence. The alignment was performed with the ClustalW2 algorithm with structure-based manual adjustment of the α4 region in GSDMA3 and GSDMD. Identical residues are highlighted by dark red background and conserved residues are coloured red. The residues involved in the autoinhibitory interactions are marked underneath the sequences with blue triangle for polar residues, orange rhombus for hydrophobic residues and black rhombus for hydrophobic residues in the second inter-domain interface. The residue number is indicated on the left of the sequence.
Extended Data Figure 9 Mutations in GSDMA3-N affecting lipid binding and pore formation also reduce pyroptosis.
a, Effects of L184D/E14K mutations on pyroptosis-inducing activity of GSDMA3-N (residues 1–284). Full-length GSDMA3 or its gasdermin-N domain (wild type or indicated mutants) was transfected into 293T cells. ATP-based cell viability is expressed as mean ± s.d. from three technical replicates. b, c, Effects of L184D/E14K mutations on lipid-binding and liposome-leakage-inducing activities of GSDMA3-N domain. Liposomes containing 80% phosphatidylcholine and 20% phosphatidylethanolamine, cardiolipin, phosphatidylinositol or PtdIns(4,5)P2 were treated with purified GSDMA3. After ultracentrifugation, the liposome-free supernatant (S) and the liposome pellet (P) were analysed by SDS–PAGE (b). Liposome leakage was monitored by measuring DPA chelating-induced fluorescence of released Tb3+ (c). Triton-X 100 treatment was used to achieve 100% leakage. d, Effects of L184D/E14K mutations on pore formation by GSDMA3-N. Representative electron microscopy images of the pores on the cardiolipin liposome are shown (scale bar, 100 nm). All data shown are representative of three independent experiments.
Supplementary information
Supplementary Information
This file contains the uncropped immunoblot images presented in figure 5 and the Extended Data Figures. (PDF 1392 kb)
Membrane targeting of the Gasdermin-N domain of GSDMD during pyroptosis
The Gasdermin-N domain of GSDMD (GSDMD-N L192D) fused N-terminal to EGFP was stably expressed in HeLa cells under a tetracycline-inducible promoter. Pyroptosis was triggered by doxycycline addition to induce GSDMD-N L192D-EGFP expression. Shown are real-time videos of two representative pyroptotic cells. Scale bar, 15 μm. The time-lapse images for Video 1 are in Fig. 1c. (MP4 9991 kb)
Membrane targeting of the Gasdermin-N domain of GSDMD during pyroptosis
The Gasdermin-N domain of GSDMD (GSDMD-N L192D) fused N-terminal to EGFP was stably expressed in HeLa cells under a tetracycline-inducible promoter. Pyroptosis was triggered by doxycycline addition to induce GSDMD-N L192D-EGFP expression. Shown are real-time videos of two representative pyroptotic cells. Scale bar, 15 μm. (MP4 9764 kb)
Membrane targeting of the Gasdermin-N domain of GSDMA during pyroptosis.
The Gasdermin-N domain of GSDMA3 (GSDMA3-N L184D) fused N-terminal to EGFP was stably expressed in HeLa cells under a tetracycline-inducible promoter. Pyroptosis was triggered by doxycycline addition to induce GSDMA3-N L184D-EGFP expression. Shown are real-time videos of two representative pyroptotic cells. Scale bar, 15 μm. The time-lapse images for Video 3 are in Extended Data Fig. 3c. (MP4 9412 kb)
Membrane targeting of the Gasdermin-N domain of GSDMA during pyroptosis.
The Gasdermin-N domain of GSDMA3 (GSDMA3-N L184D) fused N-terminal to EGFP was stably expressed in HeLa cells under a tetracycline-inducible promoter. Pyroptosis was triggered by doxycycline addition to induce GSDMA3-N L184D-EGFP expression. Shown are real-time videos of two representative pyroptotic cells. Scale bar, 15 μm. (MP4 9569 kb)
Rights and permissions
About this article
Cite this article
Ding, J., Wang, K., Liu, W. et al. Pore-forming activity and structural autoinhibition of the gasdermin family. Nature 535, 111–116 (2016). https://doi.org/10.1038/nature18590
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nature18590
This article is cited by
-
ChemR23 signaling ameliorates brain injury via inhibiting NLRP3 inflammasome-mediated neuronal pyroptosis in ischemic stroke
Journal of Translational Medicine (2024)
-
cGAS-STING, inflammasomes and pyroptosis: an overview of crosstalk mechanism of activation and regulation
Cell Communication and Signaling (2024)
-
Platycodon D protects human nasal epithelial cells from pyroptosis through the Nrf2/HO-1/ROS signaling cascade in chronic rhinosinusitis
Chinese Medicine (2024)
-
Molecular mechanism of bovine Gasdermin D-mediated pyroptosis
Veterinary Research (2024)
-
Pyroptosis: a double-edged sword in lung cancer and other respiratory diseases
Cell Communication and Signaling (2024)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.