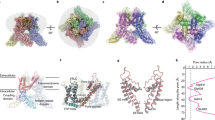
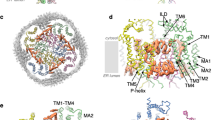
Abstract
When integral membrane proteins are visualized in detergents or other artificial systems, an important layer of information is lost regarding lipid interactions and their effects on protein structure. This is especially relevant to proteins for which lipids have both structural and regulatory roles. Here we demonstrate the power of combining electron cryo-microscopy with lipid nanodisc technology to ascertain the structure of the rat TRPV1 ion channel in a native bilayer environment. Using this approach, we determined the locations of annular and regulatory lipids and showed that specific phospholipid interactions enhance binding of a spider toxin to TRPV1 through formation of a tripartite complex. Furthermore, phosphatidylinositol lipids occupy the binding site for capsaicin and other vanilloid ligands, suggesting a mechanism whereby chemical or thermal stimuli elicit channel activation by promoting the release of bioactive lipids from a critical allosteric regulatory site.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
Accession codes
Primary accessions
Electron Microscopy Data Bank
Protein Data Bank
Data deposits
The three-dimensional cryo-EM density maps of the TRPV1–nanodisc complexes without low-pass filter and amplitude modification have been deposited in the Electron Microscopy Data Bank under accession numbers EMD-8118 (TRPV1–nanodisc), EMD-8117 (TRPV1–RTX/DkTx–nanodisc), EMD-8119 (TRPV1–capsazepine–nanodisc) and EMD-8120 (TRPV1–capsazepine in amphipol). Particle image stacks after motion correction related to TRPV1–nanodisc and TRPV1–RTX/DkTx–nanodisc have been deposited in the Electron Microscopy Pilot Image Archive (http://www.ebi.ac.uk/pdbe/emdb/empiar/) under accession number EMPIAR-10059. Atomic coordinates for the atomic model of TRPV1 in nanodisc, TRPV1–RTX/DkTx in nanodisc and TRPV1–capsazepine in nanodisc have been deposited in the Protein Data Bank under accession numbers 5IRZ, 5IRX and 5IS0.
References
Hilgemann, D. W. Getting ready for the decade of the lipids. Annu. Rev. Physiol. 65, 697–700 (2003)
Hille, B., Dickson, E. J., Kruse, M., Vivas, O. & Suh, B. C. Phosphoinositides regulate ion channels. Biochim. Biophys. Acta 1851, 844–856 (2015)
Lee, A. G. Biological membranes: the importance of molecular detail. Trends Biochem. Sci. 36, 493–500 (2011)
Caffrey, M. A lipid’s eye view of membrane protein crystallization in mesophases. Curr. Opin. Struct. Biol. 10, 486–497 (2000)
Landau, E. M. & Rosenbusch, J. P. Lipidic cubic phases: a novel concept for the crystallization of membrane proteins. Proc. Natl Acad. Sci. USA 93, 14532–14535 (1996)
Gonen, T. et al. Lipid-protein interactions in double-layered two-dimensional AQP0 crystals. Nature 438, 633–638 (2005)
Wang, L. & Sigworth, F. J. Structure of the BK potassium channel in a lipid membrane from electron cryomicroscopy. Nature 461, 292–295 (2009)
Bayburt, T. H., Grinkova, Y. V. & Sligar, S. G. Self-assembly of discoidal phospholipid bilayer nanoparticles with membrane scaffold proteins. Nano Lett. 2, 853–856 (2002)
Banerjee, S., Huber, T. & Sakmar, T. P. Rapid incorporation of functional rhodopsin into nanoscale apolipoprotein bound bilayer (NABB) particles. J. Mol. Biol. 377, 1067–1081 (2008)
Ritchie, T. K. et al. Reconstitution of membrane proteins in phospholipid bilayer nanodiscs. Methods Enzymol. 464, 211–231 (2009)
Efremov, R. G., Leitner, A., Aebersold, R. & Raunser, S. Architecture and conformational switch mechanism of the ryanodine receptor. Nature 517, 39–43 (2015)
Frauenfeld, J. et al. Cryo-EM structure of the ribosome–SecYE complex in the membrane environment. Nature Struct. Mol. Biol. 18, 614–621 (2011)
Bai, X. C., McMullan, G. & Scheres, S. H. How cryo-EM is revolutionizing structural biology. Trends Biochem. Sci. 40, 49–57 (2015)
Cheng, Y. Single-particle cryo-EM at crystallographic resolution. Cell 161, 450–457 (2015)
Kühlbrandt, W. Cryo-EM enters a new era. eLife 3, e03678 (2014)
Bevan, S., Quallo, T. & Andersson, D. A. Trpv1. Handb. Exp. Pharmacol. 222, 207–245 (2014)
Julius, D. TRP channels and pain. Annu. Rev. Cell Dev. Biol. 29, 355–384 (2013)
Cao, E., Liao, M., Cheng, Y. & Julius, D. TRPV1 structures in distinct conformations reveal activation mechanisms. Nature 504, 113–118 (2013)
Liao, M., Cao, E., Julius, D. & Cheng, Y. Structure of the TRPV1 ion channel determined by electron cryo-microscopy. Nature 504, 107–112 (2013)
Long, S. B., Tao, X., Campbell, E. B. & MacKinnon, R. Atomic structure of a voltage-dependent K+ channel in a lipid membrane-like environment. Nature 450, 376–382 (2007)
Szallasi, A. & Blumberg, P. M. Resiniferatoxin, a phorbol-related diterpene, acts as an ultrapotent analog of capsaicin, the irritant constituent in red pepper. Neuroscience 30, 515–520 (1989)
Bohlen, C. J. et al. A bivalent tarantula toxin activates the capsaicin receptor, TRPV1, by targeting the outer pore domain. Cell 141, 834–845 (2010)
Chou, M. Z., Mtui, T., Gao, Y. D., Kohler, M. & Middleton, R. E. Resiniferatoxin binds to the capsaicin receptor (TRPV1) near the extracellular side of the S4 transmembrane domain. Biochemistry 43, 2501–2511 (2004)
Gavva, N. R. et al. Molecular determinants of vanilloid sensitivity in TRPV1. J. Biol. Chem. 279, 20283–20295 (2004)
Hanson, S. M., Newstead, S., Swartz, K. J. & Sansom, M. S. P. Capsaicin interaction with TRPV1 channels in a lipid bilayer: molecular dynamics simulation. Biophys. J. 108, 1425–1434 (2015)
Jordt, S. E. & Julius, D. Molecular basis for species-specific sensitivity to “hot” chili peppers. Cell 108, 421–430 (2002)
Phillips, E., Reeve, A., Bevan, S. & McIntyre, P. Identification of species-specific determinants of the action of the antagonist capsazepine and the agonist PPAHV on TRPV1. J. Biol. Chem. 279, 17165–17172 (2004)
Yang, F. et al. Structural mechanism underlying capsaicin binding and activation of the TRPV1 ion channel. Nat. Chem. Biol. 11, 518–524 (2015)
Bevan, S. et al. Capsazepine: a competitive antagonist of the sensory neurone excitant capsaicin. Br. J. Pharmacol. 107, 544–552 (1992)
Boukalova, S., Marsakova, L., Teisinger, J. & Vlachova, V. Conserved residues within the putative S4-S5 region serve distinct functions among thermosensitive vanilloid transient receptor potential (TRPV) channels. J. Biol. Chem. 285, 41455–41462 (2010)
Lee, S. Y. & MacKinnon, R. A membrane-access mechanism of ion channel inhibition by voltage sensor toxins from spider venom. Nature 430, 232–235 (2004)
Milescu, M. et al. Interactions between lipids and voltage sensor paddles detected with tarantula toxins. Nature Struct. Mol. Biol. 16, 1080–1085 (2009)
Milescu, M. et al. Tarantula toxins interact with voltage sensors within lipid membranes. J. Gen. Physiol. 130, 497–511 (2007)
Bae, C. et al. Structural insights into the mechanism of activation of the TRPV1 channel by a membrane-bound tarantula toxin. eLife 5, e11273 (2016)
Hardie, R. C. TRP channels and lipids: from Drosophila to mammalian physiology. J. Physiol. 578, 9–24 (2007)
Qin, F. Regulation of TRP ion channels by phosphatidylinositol-4,5-bisphosphate. Handb. Exp. Pharmacol. 179, 509–525 (2007)
Rohacs, T. Phosphoinositide regulation of TRPV1 revisited. Pflugers Arch. 467, 1851–1869 (2015)
Cao, E., Cordero-Morales, J. F., Liu, B., Qin, F. & Julius, D. TRPV1 channels are intrinsically heat sensitive and negatively regulated by phosphoinositide lipids. Neuron 77, 667–679 (2013)
Prescott, E. D. & Julius, D. A modular PIP2 binding site as a determinant of capsaicin receptor sensitivity. Science 300, 1284–1288 (2003)
Ufret-Vincenty, C. A. et al. Mechanism for phosphoinositide selectivity and activation of TRPV1 ion channels. J. Gen. Physiol. 145, 431–442 (2015)
Ufret-Vincenty, C. A., Klein, R. M., Hua, L., Angueyra, J. & Gordon, S. E. Localization of the PIP2 sensor of TRPV1 ion channels. J. Biol. Chem. 286, 9688–9698 (2011)
Hansen, S. B., Tao, X. & MacKinnon, R. Structural basis of PIP2 activation of the classical inward rectifier K+ channel Kir2.2. Nature 477, 495–498 (2011)
Booth, D. S., Avila-Sakar, A. & Cheng, Y. Visualizing proteins and macromolecular complexes by negative stain EM: from grid preparation to image acquisition. J. Vis. Exp. 58, 3227 (2011)
Li, X. et al. Electron counting and beam-induced motion correction enable near-atomic-resolution single-particle cryo-EM. Nature Methods 10, 584–590 (2013)
Li, X., Zheng, S., Agard, D. A. & Cheng, Y. Asynchronous data acquisition and on-the-fly analysis of dose fractionated cryoEM images by UCSFImage. J. Struct. Biol. 192, 174–178 (2015)
Rohou, A. & Grigorieff, N. CTFFIND4: fast and accurate defocus estimation from electron micrographs. J. Struct. Biol. 192, 216–221 (2015)
Frank, J. et al. SPIDER and WEB: processing and visualization of images in 3D electron microscopy and related fields. J. Struct. Biol. 116, 190–199 (1996)
Grant, T. & Grigorieff, N. Measuring the optimal exposure for single particle cryo-EM using a 2.6 Å reconstruction of rotavirus VP6. eLife 4, e06980 (2015)
Scheres, S. H. W. RELION: implementation of a Bayesian approach to cryo-EM structure determination. J. Struct. Biol. 180, 519–530 (2012)
Scheres, S. H. W. & Chen, S. Prevention of overfitting in cryo-EM structure determination. Nature Methods 9, 853–854 (2012)
Kucukelbir, A., Sigworth, F. J. & Tagare, H. D. Quantifying the local resolution of cryo-EM density maps. Nature Methods 11, 63–65 (2014)
Hohn, M. et al. SPARX, a new environment for cryo-EM image processing. J. Struct. Biol. 157, 47–55 (2007)
Tang, G. et al. EMAN2: an extensible image processing suite for electron microscopy. J. Struct. Biol. 157, 38–46 (2007)
Pettersen, E. F. et al. UCSF Chimera—a visualization system for exploratory research and analysis. J. Comput. Chem. 25, 1605–1612 (2004)
Emsley, P., Lohkamp, B., Scott, W. G. & Cowtan, K. Features and development of Coot. Acta Crystallogr. D 66, 486–501 (2010)
Moriarty, N. W., Grosse-Kunstleve, R. W. & Adams, P. D. electronic Ligand Builder and Optimization Workbench (eLBOW): a tool for ligand coordinate and restraint generation. Acta Crystallogr. D 65, 1074–1080 (2009)
Adams, P. D. et al. PHENIX: a comprehensive Python-based system for macromolecular structure solution. Acta Crystallogr. D 66, 213–221 (2010)
van Aalten, D. M. F. et al. PRODRG, a program for generating molecular topologies and unique molecular descriptors from coordinates of small molecules. J. Comput. Aided Mol. Des. 10, 255–262 (1996)
Afonine, P. V., Headd, J. J., Terwilliger, T. C. & Adams, P. D. New tool: phenix. real_space_refine. Computational Crystallography Newsletter 4, 43–44 (2013)
Amunts, A. et al. Structure of the yeast mitochondrial large ribosomal subunit. Science 343, 1485–1489 (2014)
Chen, V. B. et al. MolProbity: all-atom structure validation for macromolecular crystallography. Acta Crystallogr. D 66, 12–21 (2010)
Acknowledgements
We thank our laboratory colleagues, past and present, for many helpful discussions and manuscript critiques, C. Paulsen and E. Green for helping with initial screening for nanodisc reconstitution, and E. Palovcak for providing scripts for focused classification. This work was supported by grants from the National Institutes of Health (R01NS047723, R37NS065071 and R01NS055299 to D.J., S10OD020054, R01GM098672, P01GM111126 and P50GM082250 to Y.C.). Y.C. is an Investigator with the Howard Hughes Medical Institute.
Author information
Authors and Affiliations
Contributions
Y.G. carried out protein purification, nanodisc reconstitution, and detailed cryo-EM experiments, including data acquisition, image processing, atomic model building and refinement of TRPV1–nanodisc complexes. E.C. carried out cryo-EM experiments of the TRPV1–capsazepine complex solubilized in amphipol. All authors contributed to experimental design, data analysis, and manuscript preparation.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Extended data figures and tables
Extended Data Figure 1 Reconstitution of TRPV1 into lipid nanodisc.
a, Size-exclusion chromatography of TRPV1 channel reconstituted into lipid nanodisc using MSP2N2. Void volume and peaks corresponding to TRPV1 and cleaved MBP are indicated. b, SDS–polyacrylamide gel electrophoresis (SDS–PAGE) of detergent-solubilized MBP–TRPV1 fusion protein and material from nanodisc reconstituted with TRPV1 following MBP cleavage (middle peak in a). Note the presence of both bands for TRPV1 and MSP2N2. c, Representative micrograph of negative-stained TRPV1–nanodisc sample showing mono-dispersed and homogeneous particles. d, Reference-free two-dimensional class averages of particles in c, revealing band-like density contributed by the lipid disc (side view) and tetrameric arrangement of channel subunits (top view). e, Two-dimensional class averages of the same protein reconstituted into MSP1E3 nanodisc, which is smaller in diameter. Note the extra space within the disc offered by MSP2N2 scaffold protein in d. The size of the class average window is 258 Å.
Extended Data Figure 2 Single-particle cryo-EM of unliganded TRPV1 in lipid nanodisc.
a, Representative raw micrograph of apo TRPV1 in nanodisc. b, Fourier transform of image in a. Note that Thon rings are visible at up to 3 Å. c, Gallery of two-dimensional class averages, with size of window as 233 Å. d, Slices through the unsharpened density map at different levels along the channel symmetry axis (numbers start from extracellular side). e, Euler angle distribution of all particles included in the calculation of the final three-dimensional reconstruction. Position of each sphere (grey) relative to the density map (green) represents its angle assignment and the radius of the sphere is proportional to the amount of particles in this specific orientation. f, Final three-dimensional density map coloured with local resolution in side and top views. g, Fourier shell coefficient (FSC) curves between two independently refined half maps before (blue) and after (red) the post-processing in RELION. h, FSC curves for cross-validation: model versus summed map (purple), model versus half map 1 (used in test refinement, cyan), model versus half map 2 (not used in test refinement, orange). Small differences between the ‘work’ and ‘free’ curves indicate little effect of over-fitting.
Extended Data Figure 3 Single-particle cryo-EM studies of agonist-bound TRPV1 in lipid nanodisc.
a, Representative raw micrograph of TRPV1–RTX/DkTx in nanodisc. b, Fourier transform of image in a. c, Gallery of two-dimensional class averages, with size of window as 233 Å. d, Slices through the unsharpened density map at different levels along the channel symmetry axis (numbers start from extracellular side). e, Euler angle distribution of all particles included in the calculation of the final three-dimensional reconstruction. Position of each sphere (grey) relative to the density map (green) represents its angle assignment and the radius of the sphere is proportional to the amount of particles in this specific orientation. f, Final three-dimensional density map coloured with local resolution in side and top views. g, FSC curves between two independently refined half maps before (blue) and after (red) the post-processing in RELION. h, FSC curves for cross-validation: model versus summed map (purple), model versus half map 1 (used in test refinement, cyan), model versus half map 2 (not used in test refinement, orange). Small differences between the ‘work’ and ‘free’ curves indicate little effect of over-fitting.
Extended Data Figure 4 Single-particle cryo-EM studies of antagonist-bound TRPV1 in lipid nanodisc.
a, Representative raw micrograph of TRPV1–capsazepine complex in nanodisc. b, Fourier transform of image in a. c, Gallery of two-dimensional class averages, with size of window as 233 Å. d, Slices through the unsharpened density map at different levels along the channel symmetry axis (numbers start from extracellular side). e, Euler angle distribution of all particles included in the calculation of the final three-dimensional reconstruction. Position of each sphere (grey) relative to the density map (green) represents its angle assignment and the radius of the sphere is proportional to the amount of particles in this specific orientation. f, Final three-dimensional density map coloured with local resolution in side and top views. g, FSC curves between two independently refined half maps before (blue) and after (red) the post-processing in RELION. h, FSC curves for cross-validation: model versus summed map (purple), model versus half map 1 (used in test refinement, cyan), model versus half map 2 (not used in test refinement, orange). Small differences between the ‘work’ and ‘free’ curves indicate little effect of over-fitting.
Extended Data Figure 5 Improved resolution for structures determined in nanodisc.
Comparison of density maps (blue mesh) determined from nanodisc- and amphipol-stabilized TRPV1 at various regions of the channel facing the lipid bilayer or at the bilayer surface. Refined atomic models (gold, nanodisc; grey, amphipol) are fit to corresponding densities. Side-chain densities were considerably improved in nanodisc-stabilized TRPV1–DkTx/RTX structure (a, b), and notable improvement was also seen for unliganded (c, d) and capsazepine-bound (e, f) channels in nanodisc.
Extended Data Figure 6 Newly resolved TRPV1 cytoplasmic region in nanodisc-stabilized structure.
a, A region in the TRPV1 C terminus, previously unresolved in amphipol-stabilized structures (blue) is clearly resolved in the nanodisc-stabilized structure. b, Enlarged view of boxed region in a showing density map (blue mesh) and superimposed model (gold). Previously resolved TRP domain and N-terminal β-strands are depicted in ribbon diagram format (cyan).
Extended Data Figure 7 Categories of lipid densities observed in TRPV1 structures.
a, Two continuous layers of density (blue) contributed by lipid head groups of bilayer within nanodisc are shown for apo channel (left) and channel in complex with RTX–DkTx (right). b, Atomic model of annular lipids could be built into well-resolved densities (blue mesh) surrounding the channel protein. DkTx is shown as ribbon diagram (pink). Top-down views show distribution of resolved annular lipids (blue) in inter-subunit crevices at the outer leaflet of the membrane. c, Well-resolved densities (blue mesh) in the structures representing a phosphatidylcholine molecule (left) and a phosphatidylinositol molecule (right). Transmembrane helices of TRPV1 close to the binding site are also shown as ribbon diagrams (grey).
Extended Data Figure 8 Focused analysis of DkTx density map.
a, Flow-chart showing procedures of focused three-dimensional classification of DkTx and proximal regions (see Methods for details). b, Atomic models for both knots of DkTx are superimposed on density maps (pink mesh).
Extended Data Figure 9 Lipid co-factor and vanilloids at the vanilloid binding site of TRPV1.
a, Chemical structure of phosphatidylinositol. b, Local environment of the phosphatidylinositol-binding site may accommodate multiple phosphatidylinositide species with phosphate substituents at the 3, 4 and/or 5 positions of the inositol ring (drawn in red). Adjacent regions of the channel are shown as ribbon diagram (grey). c, Tyr511 assumes two possible orientations that differ in apo versus agonist-bound states of the TRPV1 channel. In the apo state, one acyl chain of the resident phosphatidylinositol lipid (blue mesh superimposed with atomic model) prevents the Tyr511 side chain from assuming the upward rotamer position. d, Density maps of vanilloids (resiniferatoxin, red mesh; capsazepine, gold mesh) superimposed with density of the bound phosphatidylinositol lipid (blue mesh), suggesting that they occupy overlapping, but not identical sites. Atomic models for both drugs and their chemical structures are also shown. e, Overlap of transmembrane region of one TRPV1 subunit corresponding to apo (blue) and RTX/DkTx-bound (orange) states. Note the relatively small conformational change of the voltage sensor-like domain (S1–S4, boxed region). f, Overlap of transmembrane region of one TRPV1 subunit corresponding to apo (blue) and capsazepine-bound (gold) states.
Rights and permissions
About this article
Cite this article
Gao, Y., Cao, E., Julius, D. et al. TRPV1 structures in nanodiscs reveal mechanisms of ligand and lipid action. Nature 534, 347–351 (2016). https://doi.org/10.1038/nature17964
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nature17964
This article is cited by
-
Relation between flexibility and intrinsically disorder regions in thermosensitive TRP channels reveal allosteric effects
European Biophysics Journal (2024)
-
Recent advances in chemical protein synthesis: method developments and biological applications
Science China Chemistry (2024)
-
Release of nanodiscs from charged nano-droplets in the electrospray ionization revealed by molecular dynamics simulations
Communications Chemistry (2023)
-
Self-cyclisation as a general and efficient platform for peptide and protein macrocyclisation
Communications Chemistry (2023)
-
Structural basis of vitamin C recognition and transport by mammalian SVCT1 transporter
Nature Communications (2023)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.