Abstract
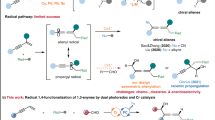
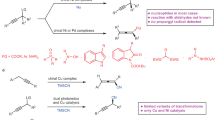
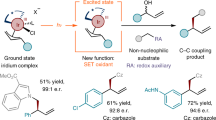
An important goal of modern organic chemistry is to develop new catalytic strategies for enantioselective carbon–carbon bond formation that can be used to generate quaternary stereogenic centres. Whereas considerable advances have been achieved by exploiting polar reactivity1, radical transformations have been far less successful2. This is despite the fact that open-shell intermediates are intrinsically primed for connecting structurally congested carbons, as their reactivity is only marginally affected by steric factors3. Here we show how the combination of photoredox4 and asymmetric organic catalysis5 enables enantioselective radical conjugate additions to β,β-disubstituted cyclic enones to obtain quaternary carbon stereocentres with high fidelity. Critical to our success was the design of a chiral organic catalyst, containing a redox-active carbazole moiety, that drives the formation of iminium ions and the stereoselective trapping of photochemically generated carbon-centred radicals by means of an electron-relay mechanism. We demonstrate the generality of this organocatalytic radical-trapping strategy with two sets of open-shell intermediates, formed through unrelated light-triggered pathways from readily available substrates and photoredox catalysts—this method represents the application of iminium ion activation6 (a successful catalytic strategy for enantioselective polar chemistry) within the realm of radical reactivity.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
Accession codes
Data deposits
Crystallographic data for the iminium ion A-1 and for compounds 3i and 7h have been deposited with the Cambridge Crystallographic Data Centre, accession numbers CCDC 1437991, 1437992 and 1437993, respectively.
References
Quasdorf, K. W. & Overman, L. E. Catalytic enantioselective synthesis of quaternary carbon stereocentres. Nature 516, 181–191 (2014)
Murakata, M., Jono, T., Mizuno, Y. & Hoshino, O. Construction of chiral quaternary carbon centers by catalytic enantioselective radical-mediated allylation of α-iodolactones using allyltributyltin in the presence of a chiral Lewis acid. J. Am. Chem. Soc. 119, 11713–11714 (1997)
Fischer, H. & Radom, L. Factors controlling the addition of carbon-centered radicals to alkenes — an experimental and theoretical perspective. Angew. Chem. Int. Ed. 40, 1340–1371 (2001)
Schultz, D. M. & Yoon, T. P. Solar synthesis: prospects in visible light photocatalysis. Science 343, 1239176 (2014)
MacMillan, D. W. C. The advent and development of organocatalysis. Nature 455, 304–308 (2008)
Lelais, G. & MacMillan, D. W. C. Modern strategies in organic catalysis: the advent and development of iminium activation. Aldrichim. Acta 39, 79–87 (2006)
Hawner, C. & Alexakis, A. Metal-catalyzed asymmetric conjugate addition reaction: formation of quaternary stereocenters. Chem. Commun. 46, 7295–7306 (2010)
Hird, A. W. & Hoveyda, A. H. Catalytic enantioselective alkylations of tetrasubstituted olefins. Synthesis of all-carbon quaternary stereogenic centers through Cu-catalyzed asymmetric conjugate additions of alkylzinc reagents to enones. J. Am. Chem. Soc. 127, 14988–14989 (2005)
Shintani, R., Tsutsumi, Y., Nagaosa, M., Nishimura, T. & Hayashi, T. Sodium tetraarylborates as effective nucleophiles in rhodium/diene-catalyzed 1,4-addition to β,β-disubstituted α,β-unsaturated ketones: catalytic asymmetric construction of quaternary carbon stereocenters. J. Am. Chem. Soc. 131, 13588–13589 (2009)
Liu, Y., Han, S.-J., Liu, W.-B. & Stoltz, B. M. Catalytic enantioselective construction of quaternary stereocenters: assembly of key building blocks for the synthesis of biologically active molecules. Acc. Chem. Res. 48, 740–751 (2015)
Sidera, M., Roth, P. M. C., Maksymowicz, R. M. & Fletcher, S. P. Formation of quaternary centers by copper-catalyzed asymmetric conjugate addition of alkylzirconium reagents. Angew. Chem. Int. Ed. 52, 7995–7999 (2013)
Srikanth, G. S. C. & Castle, S. L. Advances in radical conjugate additions. Tetrahedron 61, 10377–10441 (2005)
Sibi, M. P., Ji, J., Wu, J. H., Gürtler, S. & Porter, N. A. Chiral Lewis acid catalysis in radical reactions: enantioselective conjugate radical additions. J. Am. Chem. Soc. 118, 9200–9201 (1996)
Gansäuer, A., Lauterbach, T., Bluhm, H. & Noltemeyer, M. A catalytic enantioselective electron transfer reaction: titanocene-catalyzed enantioselective formation of radicals from meso-epoxides. Angew. Chem. Int. Ed. 38, 2909–2910 (1999)
Ruiz Espelt, L., McPherson, I. S., Wiesnsch, E. M. & Yoon, T. P. Enantioselective conjugate additions of α-amino radicals via cooperative photoredox and Lewis acid catalysis. J. Am. Chem. Soc. 137, 2452–2455 (2015)
Damm, W. et al. Diastereofacial selectivity in reactions of substituted cyclohexyl radicals. An experimental and theoretical study. J. Am. Chem. Soc. 114, 4067–4079 (1992)
Schnermann, M. J. & Overman, L. E. A concise synthesis of (−)-Aplyviolene facilitated by a strategic tertiary radical conjugate addition. Angew. Chem. Int. Ed. 51, 9576–9580 (2012)
Prier, C. K., Rankic, D. A. & MacMillan, D. W. C. Visible light photoredox catalysis with transition metal complexes: applications in organic synthesis. Chem. Rev. 113, 5322–5363 (2013)
Gu, X. et al. A general, scalable, organocatalytic nitro-Michael addition to enones: enantioselective access to all-carbon quaternary stereocenters. Org. Lett. 17, 1505–1508 (2015)
Poniatowski, A. J. & Floreancig, P. E. in Carbon-Centered Free Radicals and Radical Cations: Structure, Reactivity, and Dynamics Ch. 3 (ed. Forbes, M. D. E. ) 43–49 (Wiley & Sons, 2010)
Jakobsen, H. J., Lawesson, S. O., Marshall, J. T. B., Schroll, G. & Williams, D. H. Mass spectrometry. XII. Mass spectra of enamines. J. Chem. Soc. B 940–946 (1966)
Page, C. C., Moser, C. C., Chen, X. & Dutton, P. L. Natural engineering principles of electron tunnelling in biological oxidation-reduction. Nature 402, 47–52 (1999)
Häfelinger, G. & Mack, H.-G. in The Chemistry of Enamines Ch. 1 (ed. Rappaport, Z. ) 1–87 (Wiley & Sons, 1994)
Okada, Y., Nishimoto, A., Akaba, R. & Chiba, K. Electron-transfer-induced intermolecular [2+2] cycloaddition reactions based on the aromatic “redox tag” strategy. J. Org. Chem. 76, 3470–3476 (2011)
Tzirakis, M. D., Lykakis, I. N. & Orfanopoulos, M. Decatungstate as an efficient photocatalyst in organic chemistry. Chem. Soc. Rev. 38, 2609–2621 (2009)
Ravelli, D., Albini, A. & Fagnoni, M. Smooth photocatalytic preparation of 2-substituted 1,3-benzodioxoles. Chem. Eur. J. 17, 572–579 (2011)
Prudhomme, D. R., Wang, Z. & Rizzo, C. J. An improved photosensitizer for the photoinduced electron-transfer deoxygenation of benzoates and m-(trifluoromethyl)benzoates. J. Org. Chem. 62, 8257–8260 (1997)
Blouin, N. & Leclerc, M. Poly(2,7-carbazole)s: structure-property relationships. Acc. Chem. Res. 41, 1110–1119 (2008)
Yamase, T., Takabayashi, N. & Kaji, M. Solution photochemistry of tetrakis(tetrabutylammonium) decatungstate(VI) and catalytic hydrogen evolution from alcohols. J. Chem. Soc. Dalton Trans. 793–799 (1984)
Bertrand, S., Hoffmann, N. & Pete, J.-P. Highly efficient and stereoselective radical addition of tertiary amines to electron-deficient alkenes — application to the enantioselective synthesis of Necine bases. Eur. J. Org. Chem. 2227–2238 (2000)
Acknowledgements
Financial support was provided by the ICIQ Foundation, MINECO (project CTQ2013-45938-P and Severo Ochoa Excellence Accreditation 2014-2018, SEV-2013-0319), AGAUR (2014 SGR 1059), and the European Research Council (ERC 278541, ORGA-NAUT). J.J.M. and S.P. thank the Marie Curie COFUND (291787-ICIQ-IPMP) and the CELLEX Foundation, respectively, for postdoctoral fellowships. We thank M. Minozzi, M. Nappi and E. Raluy for preliminary investigations, D. Merli and D. Dondi for assistance with EPR experiments, and D. Ravelli for discussions.
Author information
Authors and Affiliations
Contributions
J.J.M., D.B. and S.P. performed the experiments and analysed the data. J.J.M., D.B., S.P., and P.M. designed the experiments. M.F. and P.M. conceived the project. P.M. directed the project, and P.M. and J.J.M. wrote the manuscript with contributions from all the authors.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Information
This file contains Supplementary Text and Data, Supplementary Figures, Supplementary Tables and additional references (see Contents for details). (PDF 10677 kb)
Rights and permissions
About this article
Cite this article
Murphy, J., Bastida, D., Paria, S. et al. Asymmetric catalytic formation of quaternary carbons by iminium ion trapping of radicals. Nature 532, 218–222 (2016). https://doi.org/10.1038/nature17438
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nature17438
This article is cited by
-
Dioxygen compatible electron donor-acceptor catalytic system and its enabled aerobic oxygenation
Nature Communications (2024)
-
Metal-free photoinduced C(sp3)–H/C(sp3)–H cross-coupling to access α‑tertiary amino acid derivatives
Nature Communications (2023)
-
A general copper-catalysed enantioconvergent radical Michaelis–Becker-type C(sp3)–P cross-coupling
Nature Synthesis (2023)
-
Reduction of unactivated alkyl chlorides enabled by light-induced single electron transfer
Science China Chemistry (2023)
-
New strategies for asymmetric photocatalysis: asymmetric organocatalytic/photoredox relay catalysis for efficient synthesis of polycyclic compounds containing vicinal amino alcohols
Science China Chemistry (2023)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.