Abstract
Macroautophagy (hereafter referred to as autophagy) is a catabolic membrane trafficking process that degrades a variety of cellular constituents and is associated with human diseases1,2,3. Although extensive studies have focused on autophagic turnover of cytoplasmic materials, little is known about the role of autophagy in degrading nuclear components. Here we report that the autophagy machinery mediates degradation of nuclear lamina components in mammals. The autophagy protein LC3/Atg8, which is involved in autophagy membrane trafficking and substrate delivery4,5,6, is present in the nucleus and directly interacts with the nuclear lamina protein lamin B1, and binds to lamin-associated domains on chromatin. This LC3–lamin B1 interaction does not downregulate lamin B1 during starvation, but mediates its degradation upon oncogenic insults, such as by activated RAS. Lamin B1 degradation is achieved by nucleus-to-cytoplasm transport that delivers lamin B1 to the lysosome. Inhibiting autophagy or the LC3–lamin B1 interaction prevents activated RAS-induced lamin B1 loss and attenuates oncogene-induced senescence in primary human cells. Our study suggests that this new function of autophagy acts as a guarding mechanism protecting cells from tumorigenesis.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Levine, B. & Kroemer, G. Autophagy in the pathogenesis of disease. Cell 132, 27–42 (2008)
Mizushima, N., Levine, B., Cuervo, A. M. & Klionsky, D. J. Autophagy fights disease through cellular self-digestion. Nature 451, 1069–1075 (2008)
Choi, A. M., Ryter, S. W. & Levine, B. Autophagy in human health and disease. N. Engl. J. Med. 368, 651–662 (2013)
Kabeya, Y. et al. LC3, a mammalian homologue of yeast Apg8p, is localized in autophagosome membranes after processing. EMBO J. 19, 5720–5728 (2000)
Mizushima, N., Yoshimori, T. & Ohsumi, Y. The role of Atg proteins in autophagosome formation. Annu. Rev. Cell Dev. Biol. 27, 107–132 (2011)
Rogov, V., Dötsch, V., Johansen, T. & Kirkin, V. Interactions between autophagy receptors and ubiquitin-like proteins form the molecular basis for selective autophagy. Mol. Cell 53, 167–178 (2014)
Drake, K. R., Kang, M. & Kenworthy, A. K. Nucleocytoplasmic distribution and dynamics of the autophagosome marker EGFP-LC3. PLoS One 5, e9806 (2010)
Huang, R. et al. Deacetylation of nuclear LC3 drives autophagy initiation under starvation. Mol. Cell 57, 456–466 (2015)
Simon, H. U., Yousefi, S., Schmid, I. & Friis, R. ATG5 can regulate p53 expression and activation. Cell Death Dis. 5, e1339 (2014)
Lee, I. H. et al. Atg7 modulates p53 activity to regulate cell cycle and survival during metabolic stress. Science 336, 225–228 (2012)
Shimi, T. et al. The A- and B-type nuclear lamin networks: microdomains involved in chromatin organization and transcription. Genes Dev. 22, 3409–3421 (2008)
Kerppola, T. K. Bimolecular fluorescence complementation(BiFC) analysis as a probe of protein interactions in living cells. Annu. Rev. Biophys. 37, 465–487 (2008)
Guelen, L. et al. Domain organization of human chromosomes revealed by mapping of nuclear lamina interactions. Nature 453, 948–951 (2008)
Lund, E., Oldenburg, A. R. & Collas, P. Enriched domain detector: a program for detection of wide genomic enrichment domains robust against local variations. Nucleic Acids Res. 42, e92 (2014)
Sadaie, M. et al. Redistribution of the Lamin B1 genomic binding profile affects rearrangement of heterochromatic domains and SAHF formation during senescence. Genes Dev. 27, 1800–1808 (2013)
Shah, P. P. et al. Lamin B1 depletion in senescent cells triggers large-scale changes in gene expression and the chromatin landscape. Genes Dev. 27, 1787–1799 (2013)
Shimi, T. et al. The role of nuclear lamin B1 in cell proliferation and senescence. Genes Dev. 25, 2579–2593 (2011)
Freund, A., Laberge, R. M., Demaria, M. & Campisi, J. Lamin B1 loss is a senescence-associated biomarker. Mol. Biol. Cell 23, 2066–2075 (2012)
Ivanov, A. et al. Lysosome-mediated processing of chromatin in senescence. J. Cell Biol. 202, 129–143 (2013)
Serrano, M., Lin, A. W., McCurrach, M. E., Beach, D. & Lowe, S. W. Oncogenic ras provokes premature cell senescence associated with accumulation of p53 and p16INK4a. Cell 88, 593–602 (1997)
Collado, M., Blasco, M. A. & Serrano, M. Cellular senescence in cancer and aging. Cell 130, 223–233 (2007)
Young, A. R. et al. Autophagy mediates the mitotic senescence transition. Genes Dev. 23, 798–803 (2009)
Liu, H. et al. Down-regulation of autophagy-related protein 5(ATG5) contributes to the pathogenesis of early-stage cutaneous melanoma. Sci. Transl. Med. 5, 202ra123 (2013)
Roberts, P. et al. Piecemeal microautophagy of nucleus in Saccharomyces cerevisiae . Mol. Biol. Cell 14, 129–141 (2003)
Mizushima, N., Yoshimori, T. & Levine, B. Methods in mammalian autophagy research. Cell 140, 313–326 (2010)
Pankiv, S. et al. p62/SQSTM1 binds directly to Atg8/LC3 to facilitate degradation of ubiquitinated protein aggregates by autophagy. J. Biol. Chem. 282, 24131–24145 (2007)
Toyama, B. H. et al. Identification of long-lived proteins reveals exceptional stability of essential cellular structures. Cell 154, 971–982 (2013)
Mochida, K. et al. Receptor-mediated selective autophagy degrades the endoplasmic reticulum and the nucleus. Nature 522, 359–362 (2015)
Butin-Israeli, V. et al. Role of lamin B1 in chromatin instability. Mol. Cell. Biol. 35, 884–898 (2015)
Dreesen, O. et al. Lamin B1 fluctuations have differential effects on cellular proliferation and senescence. J. Cell Biol. 200, 605–617 (2013)
Dou, Z. et al. Class IA PI3K p110β subunit promotes autophagy through Rab5 small GTPase in response to growth factor limitation. Mol. Cell 50, 29–42 (2013)
Wiederschain, D. et al. Single-vector inducible lentiviral RNAi system for oncology target validation. Cell Cycle 8, 498–504 (2009)
Kirkin, V. et al. A role for NBR1 in autophagosomal degradation of ubiquitinated substrates. Mol. Cell 33, 505–516 (2009)
Pan, J. A., Ullman, E., Dou, Z. & Zong, W. X. Inhibition of protein degradation induces apoptosis through a microtubule-associated protein 1 light chain 3-mediated activation of caspase-8 at intracellular membranes. Mol. Cell. Biol. 31, 3158–3170 (2011)
Zhong, Y. et al. Distinct regulation of autophagic activity by Atg14L and Rubicon associated with Beclin 1–phosphatidylinositol-3-kinase complex. Nature Cell Biol. 11, 468–476 (2009)
N’Diaye, E. N. et al. PLIC proteins or ubiquilins regulate autophagy-dependent cell survival during nutrient starvation. EMBO Rep. 10, 173–179 (2009)
Larkin, M. A. et al. Clustal W and Clustal X version 2.0. Bioinformatics 23, 2947–2948 (2007)
Chandra, T. et al. Independence of repressive histone marks and chromatin compaction during senescent heterochromatic layer formation. Mol. Cell 47, 203–214 (2012)
Acknowledgements
We thank members of the Berger, Adams, and Goldman laboratories for technical assistance and discussions. We acknowledge A. L. Stout for help with confocal microscopy, and the electron microscopy resource laboratory for assistance on TEM. We thank Z. Yue for sharing the GFP antibody and reading the manuscript, and M. Narita and R. Salama for help with LADs definition. Z.D. is supported by a fellow award from the Leukemia & Lymphoma Society. B.C.C. is supported by career development awards from the Dermatology Foundation, Melanoma Research Foundation, and American Skin Association. S.L.B., P.D.A. and R.M. are supported by NIA P01 grant (P01AG031862). S.L.B. is also supported by NIH R01 CA078831. R.D.G. is supported by R01 GM106023 and the Progeria Research Foundation.
Author information
Authors and Affiliations
Contributions
Z.D., A.I., P.D.A., and S.L.B. conceived the project. Z.D. performed most of the experiments. C.X., G.D., B.C.C., A.M.D., and P.P.S. performed and analysed ChIP-seq. T.S. performed three-dimensional structural illumination microscopy imaging. T.S., S.A.A., and R.D.G. contributed novel lamin reagents and experimental design. J.-A.P., J.M.C., and W.-X.Z. contributed novel autophagy and senescence reagents. J.Z. performed Atg7 knockdown. M.D.R. and R.M. contributed to the biochemistry characterization of LC3–lamin B1 interaction. T.L. and T.J. contributed novel autophagy constructs and experimental design. Z.D., P.D.A., and S.L.B. composed the manuscript. All authors reviewed the manuscript and discussed the work.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Extended data figures and tables
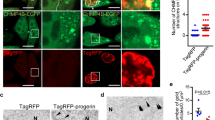
Extended Data Figure 1 Characterization of LC3 and lamin B1 association.
a, Protein gel staining of purified lamin B1 protein. b, c, Purified lamin B1 protein was subjected to GST pull-down. d, Endogenous LC3 immunoprecipitation in HEK293T cells. e, IMR90 stably expressing GFP–LC3 constructs were starved and imaged. f, Endogenous co-IP in wild-type and Atg5 knockout mouse embryonic fibroblasts. g, Nuclear fractions of control and Atg7 knockdown IMR90 cells were analysed by LC3 immunoprecipitation. h–j, BiFC analysis of LC3–lamin B1 interaction. HeLa cells were transfected with the indicated combination of split Venus constructs and analysed as follows. h, Cells were fixed and imaged. i, Lysates were analysed by immunoblotting. j, Cells were scored for Venus positivity. Bars, mean ± s.d.; n = 4, with over 500 cells; *P < 0.001; unpaired two-tailed Student’s t-test.
Extended Data Figure 2 LC3 interacts with LADs on chromatin.
a, b, ChIP–qPCR of proliferating IMR90. c, ChIP–qPCR of LC3 knockdown IMR90. Bars, mean ± s.e.m. (a, b), s.d. (c); n = 3; *P < 0.05, **P < 0.005, ***P < 0.0001; NS, non-significant; unpaired two-tailed Student’s t-test. d–i, ChIP-sequencing analyses. d, Related to Fig. 2c, a zoom-in window of chromosome 3. e, f, Analyses of two replicates at LADs and LC3ADs. g, Per-nucleotide overlap of published data sets with the LADs called from this study. Number unit: megabases. h, Enrichment over LC3ADs. *P < 2.2 × 10−16; one-sided Wilcoxon test. i, Analysis of our lamin B1 and LC3 ChIP-seq at LADs defined by other studies, and randomly sampled non-LAD loci (Ctrl). *P < 2.2 × 10−16; one-sided Wilcoxon test.
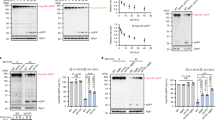
Extended Data Figure 3 Lamin B1 degradation upon HRasV12-induced senescence.
a, Related to Fig. 3b. Immunoblotting of immortalized IMR90. b, GFP–lamin B1 stably expressing IMR90 cells were treated as indicated and imaged. Cytoplasmic signals are indicated by arrows. c–e, TEM analyses of IMR90. Nu, nucleus. f, IMR90 cells stably expressing mCherry–GFP–lamin B1 were imaged and quantified. g, Cells as in f were treated with bafilomycin A1 and imaged under confocal microscopy.
Extended Data Figure 4 Imaging analyses of mCherry–GFP–lamin B1 HRasV12 cells.
a, Related to Fig. 3c. mCherry–GFP–lamin B1 HRasV12 cells stably expressing IMR90 were imaged by three-dimensional super-resolution microscopy. Sections shown span the top, middle, and bottom layers of the cell. The mCherry channel was deliberately under-exposed to prevent over-saturation of the cytoplasmic signals. Scale bar, 5 μm. The insets are presented in Fig. 3c. b, Live-cell imaging of mCherry–GFP–lamin B1 HRasV12 IMR90. Images shown are the maximum-projection combining all z-sections. Nucleus-to-cytoplasm transport events are labelled sequentially as indicated. Note the initial yellow signal, followed by disappearance of GFP then mCherry, in events 1 and 3; event 2 was not yet degraded by the end of the imaging.
Extended Data Figure 5 CCF and lamin B1 are targeted by autophagy.
a, b, IMR90 cells stably expressing GFP–LC3 and HRasV12 were stained with indicated antibodies and imaged under confocal microscopy. Cytoplasmic events are labelled by arrows. c, HRasV12 IMR90 cells were stained with LC3 antibody. d, Related to Fig. 3e, immuno-TEM analysis of GFP–lamin B1 IMR90 cells. Cells were stained with a GFP antibody and conjugated with 10 nm gold particles. Gold particles are indicated by arrows.
Extended Data Figure 6 Knockdown of Atg7 attenuates lamin B1 downregulation.
a, Related to Fig. 4a, quantification of lamin B1 immunoblots. Bars, mean ± s.e.m.; n = 3; *P < 0.05, **P < 0.005, ***P < 0.0001, compared with sh-NTC day 0; NS, non-significant. b, Reverse transcribed qPCR of cells as in Fig. 4a. Data are the mean normalized to GAPDH ± s.e.m.; n = 3. c, d, IMR90 cells were treated as indicated and analysed by immunoblotting. e, BJ cells were treated with etoposide and analysed by immunoblotting. f, g, Atg7 knockdown inhibits mCherry–GFP–lamin B1 nucleus-to-cytoplasm transport. Bars are mean ± s.d.; n = 4, over 100 cells; *P < 0.0001. h, i, ER:HRasV12 BJ cells stably expressing Dox-inducible GFP or GFP–lamin B1 were either left uninduced (bars 1 and 2), or induced with 4-OHT for 3 weeks (3–6). Cells were then induced with Dox (in the presence of 4-OHT) for an additional 2 weeks (5 and 6). i, Quantification of β-gal positivity. Bars, mean ± s.d.; n = 4, over 200 cells. j, Related to Fig. 4a, quantification of p16 immunoblots. Bars, mean ± s.e.m.; n = 3; *P < 0.05, compared with corresponding sh-NTC controls. k, ER:HRas IMR90 cells were scored for β-gal positivity. Bars, mean ± s.d.; n = 4, over 200 cells; *P < 0.0005, **P < 0.0001. One-way ANOVA coupled with Tukey’s post hoc test for a and i; all other tests were unpaired two-tailed Student’s t-tests.
Extended Data Figure 7 LC3 R10 and R11 are essential for lamin B1 binding.
a, b, HEK293T cells were transfected as indicated and analysed by co-IP. c–e, BiFC analyses in HeLa cells transfected with the indicated combination of split Venus constructs. Bars, mean ± s.d.; n = 4, over 500 cells; *P < 0.0001. f, IMR90 cells stably expressing the indicated constructs were analysed by Flag ChIP. Bars, mean ± s.e.m.; *P < 0.05, **P < 0.005; unpaired two-tailed Student’s t-test for e and f. g, LC3 R10 and R11 are necessary for co-localization with CCF in HRasV12 IMR90. CCFs are indicated with arrows.
Extended Data Figure 8 Mapping of LC3–lamin B1 interaction.
a, HEK293T cells transfected with indicated constructs were analysed by GST–LC3B pull-down. b, c, In vitro translated constructs were subjected to GST–LC3B pull-down. d, e, Evolutionary analyses of vertebrate lamin B1 and the corresponding regions of other lamin isoforms. e, Number of conserved residues normalized to total residues. f, Bacterially purified fragments were analysed by GST–LC3B pull-down. g, mCherry–GFP–lamin B1 370–458 localizes to the nucleus. h, Cells were starved and analysed by immunoblotting. i, j, Related to Fig. 4f, quantification of lamin B1 and p16 immunoblots; n = 3. k, ER-HRasV12 IMR90 cells were scored for β-gal positivity; n = 4, over 200 cells. Bars, mean ± s.e.m. (i, j), s.d. (k); NS, non-significant; *P < 0.05; **P < 0.0005; ***P < 0.0001; unpaired two-tailed Student’s t-test.
Extended Data Figure 9 Additional characterization of lamin B1 substitution mutant.
a–f, Related to Fig. 5a, in vitro translated proteins were analysed by GST–LC3B pull-down. g, LC3 immunoprecipitation in HEK293T cells transfected as indicated. The remaining interaction with the mutant is probably due to the endogenous lamin B1 that interacts with LC3 and the mutant, as shown in j. h, i, IMR90 cells were imaged under confocal microscopy and quantified. Bars, mean ± s.d.; n = 4, over 200 cells; *P < 0.05, **P < 0.005, ***P < 0.0001; unpaired two-tailed Student’s t-test. j, HEK293T transfected cells were analysed by immunoprecipitation. k, ER:HRasV12 IMR90 cells were induced with OHT and harvested for immunoblotting. l, IMR90 cells were quantified for β-gal positivity. Bars, mean ± s.d.; n = 4, over 200 cells; *P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001, NS, non-significant; one-way ANOVA coupled with Tukey’s post hoc test.
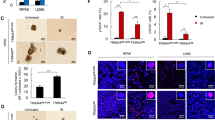
Extended Data Figure 10 Lamin B1 370–458 fragment extends cellular lifespan.
a, In vitro translated proteins were analysed by GST–LC3B pull-down. b, ER:HRasV12 IMR90 cells were quantified for β-gal positivity. Bars, mean ± s.d.; n = 4, over 200 cells; *P < 0.05; NS, non-significant; one-way ANOVA coupled with Tukey’s post hoc test. c, d, Related to Fig. 5f, representative images of β-gal. e, Related to Fig. 5g, cells were fixed and stained with DAPI. CCFs are indicated by arrows.
Supplementary information
Supplementary Information
This file contains original western blot images for Figures 1-5. (PDF 735 kb)
Rights and permissions
About this article
Cite this article
Dou, Z., Xu, C., Donahue, G. et al. Autophagy mediates degradation of nuclear lamina. Nature 527, 105–109 (2015). https://doi.org/10.1038/nature15548
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nature15548
This article is cited by
-
Histone acetylation in an Alzheimer’s disease cell model promotes homeostatic amyloid-reducing pathways
Acta Neuropathologica Communications (2024)
-
The relationship between autophagy and respiratory viruses
Archives of Microbiology (2024)
-
Autophagy modulators influence the content of important signalling molecules in PS-positive extracellular vesicles
Cell Communication and Signaling (2023)
-
Autophagy, a critical element in the aging male reproductive disorders and prostate cancer: a therapeutic point of view
Reproductive Biology and Endocrinology (2023)
-
Intrinsically disordered domain of transcription factor TCF-1 is required for T cell developmental fidelity
Nature Immunology (2023)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.