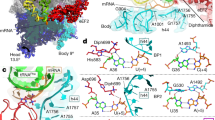
Abstract
Termination of protein synthesis occurs when a translating ribosome encounters one of three universally conserved stop codons: UAA, UAG or UGA. Release factors recognize stop codons in the ribosomal A-site to mediate release of the nascent chain and recycling of the ribosome. Bacteria decode stop codons using two separate release factors with differing specificities for the second and third bases1. By contrast, eukaryotes rely on an evolutionarily unrelated omnipotent release factor (eRF1) to recognize all three stop codons2. The molecular basis of eRF1 discrimination for stop codons over sense codons is not known. Here we present cryo-electron microscopy (cryo-EM) structures at 3.5–3.8 Å resolution of mammalian ribosomal complexes containing eRF1 interacting with each of the three stop codons in the A-site. Binding of eRF1 flips nucleotide A1825 of 18S ribosomal RNA so that it stacks on the second and third stop codon bases. This configuration pulls the fourth position base into the A-site, where it is stabilized by stacking against G626 of 18S rRNA. Thus, eRF1 exploits two rRNA nucleotides also used during transfer RNA selection to drive messenger RNA compaction. In this compacted mRNA conformation, stop codons are favoured by a hydrogen-bonding network formed between rRNA and essential eRF1 residues that constrains the identity of the bases. These results provide a molecular framework for eukaryotic stop codon recognition and have implications for future studies on the mechanisms of canonical and premature translation termination3,4.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Scolnick, E., Tompkins, R., Caskey, T. & Nirenberg, M. Release factors differing in specificity for terminator codons. Proc. Natl Acad. Sci. USA 61, 768–774 (1968)
Frolova, L. et al. A highly conserved eukaryotic protein family possessing properties of polypeptide chain release factor. Nature 372, 701–703 (1994)
Dever, T. E. & Green, R. The elongation, termination, and recycling phases of translation in eukaryotes. Cold Spring Harb. Perspect. Biol. 4, a013706 (2012)
Jackson, R. J., Hellen, C. U. T. & Pestova, T. V. Termination and post-termination events in eukaryotic translation. Adv. Protein Chem. Struct. Biol. 86, 45–93 (2012)
Muhs, M. et al. Cryo-EM of ribosomal 80S complexes with termination factors reveals the translocated cricket paralysis virus IRES. Mol. Cell 57, 422–432 (2015)
Taylor, D. et al. Cryo-EM structure of the mammalian eukaryotic release factor eRF1–eRF3-associated termination complex. Proc. Natl Acad. Sci. USA 109, 18413–18418 (2012)
Pisarev, A. V. et al. The role of ABCE1 in eukaryotic posttermination ribosomal recycling. Mol. Cell 37, 196–210 (2010)
Shoemaker, C. J. & Green, R. Kinetic analysis reveals the ordered coupling of translation termination and ribosome recycling in yeast. Proc. Natl Acad. Sci. USA 108, E1392–E1398 (2011)
Frolova, L. Y. et al. Mutations in the highly conserved GGQ motif of class 1 polypeptide release factors abolish ability of human eRF1 to trigger peptidyl-tRNA hydrolysis. RNA 5, 1014–1020 (1999)
Preis, A. et al. Cryoelectron microscopic structures of eukaryotic translation termination complexes containing eRF1-eRF3 or eRF1-ABCE1. Cell Rep. 8, 59–65 (2014)
Song, H. et al. The crystal structure of human eukaryotic release factor eRF1—mechanism of stop codon recognition and peptidyl-tRNA hydrolysis. Cell 100, 311–321 (2000)
Laurberg, M. et al. Structural basis for translation termination on the 70S ribosome. Nature 454, 852–857 (2008)
Weixlbaumer, A. et al. Insights into translational termination from the structure of RF2 bound to the ribosome. Science 322, 953–956 (2008)
Korostelev, A. et al. Crystal structure of a translation termination complex formed with release factor RF2. Proc. Natl Acad. Sci. USA 105, 19684–19689 (2008)
Brown, C. M., Stockwell, P. A., Trotman, C. N. & Tate, W. P. Sequence analysis suggests that tetra-nucleotides signal the termination of protein synthesis in eukaryotes. Nucleic Acids Res. 18, 6339–6345 (1990)
Shirokikh, N. E. et al. Quantitative analysis of ribosome–mRNA complexes at different translation stages. Nucleic Acids Res. 38, e15 (2010)
Kryuchkova, P. et al. Two-step model of stop codon recognition by eukaryotic release factor eRF1. Nucleic Acids Res. 41, 4573–4586 (2013)
Poole, E. S., Brown, C. M. & Tate, W. P. The identity of the base following the stop codon determines the efficiency of in vivo translational termination in Escherichia coli . EMBO J. 14, 151–158 (1995)
Chavatte, L., Seit-Nebi, A., Dubovaya, V. & Favre, A. The invariant uridine of stop codons contacts the conserved NIKSR loop of human eRF1 in the ribosome. EMBO J. 21, 5302–5311 (2002)
Bulygin, K. N. et al. Three distinct peptides from the N domain of translation termination factor eRF1 surround stop codon in the ribosome. RNA 16, 1902–1914 (2010)
Frolova, L., Seit-Nebi, A. & Kisselev, L. Highly conserved NIKS tetrapeptide is functionally essential in eukaryotic translation termination factor eRF1. RNA 8, 129–136 (2002)
Feng, T. et al. Optimal translational termination requires C4 lysyl hydroxylation of eRF1. Mol. Cell 53, 645–654 (2014)
Kolosov, P. et al. Invariant amino acids essential for decoding function of polypeptide release factor eRF1. Nucleic Acids Res. 33, 6418–6425 (2005)
Wong, L. E., Li, Y., Pillay, S., Frolova, L. & Pervushin, K. Selectivity of stop codon recognition in translation termination is modulated by multiple conformations of GTS loop in eRF1. Nucleic Acids Res. 40, 5751–5765 (2012)
Cheng, Z. et al. Structural insights into eRF3 and stop codon recognition by eRF1. Genes Dev. 23, 1106–1118 (2009)
Seit-Nebi, A., Frolova, L. & Kisselev, L. Conversion of omnipotent translation termination factor eRF1 into ciliate-like UGA-only unipotent eRF1. EMBO Rep. 3, 881–886 (2002)
Czaplinski, K. et al. The surveillance complex interacts with the translation release factors to enhance termination and degrade aberrant mRNAs. Genes Dev. 12, 1665–1677 (1998)
Keeling, K. M., Xue, X., Gunn, G. & Bedwell, D. M. Therapeutics based on stop codon readthrough. Annu. Rev. Genomics Hum. Genet. 15, 371–394 (2014)
Mort, M., Ivanov, D., Cooper, D. N. & Chuzhanova, N. A. A meta-analysis of nonsense mutations causing human genetic disease. Hum. Mutat. 29, 1037–1047 (2008)
Shao, S., von der Malsburg, K. & Hegde, R. S. Listerin-dependent nascent protein ubiquitination relies on ribosome subunit dissociation. Mol. Cell 50, 637–648 (2013)
Sharma, A., Mariappan, M., Appathurai, S. & Hegde, R. S. in Protein Secretion 619, 339–363 (Humana Press, 2010)
Shao, S. & Hegde, R. S. Reconstitution of a minimal ribosome-associated ubiquitination pathway with purified factors. Mol. Cell 55, 880–890 (2014)
Bai, X.-C., Fernandez, I. S., McMullan, G. & Scheres, S. H. W. Ribosome structures to near-atomic resolution from thirty thousand cryo-EM particles. eLife 2, e00461 (2013)
Li, X. et al. Electron counting and beam-induced motion correction enable near-atomic-resolution single-particle cryo-EM. Nature Methods 10, 584–590 (2013)
Scheres, S. H. W. Semi-automated selection of cryo-EM particles in RELION-1.3. J. Struct. Biol. 189, 114–122 (2015)
Scheres, S. H. W. RELION: Implementation of a Bayesian approach to cryo-EM structure determination. J. Struct. Biol. 180, 519–530 (2012)
Scheres, S. H. Beam-induced motion correction for sub-megadalton cryo-EM particles. eLife 3, e03665 (2014)
Chen, S. et al. High-resolution noise substitution to measure overfitting and validate resolution in 3D structure determination by single particle electron cryomicroscopy. Ultramicroscopy 135, 24–35 (2013)
Rosenthal, P. B. & Henderson, R. Optimal determination of particle orientation, absolute hand, and contrast loss in single-particle electron cryomicroscopy. J. Mol. Biol. 333, 721–745 (2003)
Kucukelbir, A., Sigworth, F. J. & Tagare, H. D. Quantifying the local resolution of cryo-EM density maps. Nature Methods 11, 63–65 (2014)
Voorhees, R. M., Fernandez, I. S., Scheres, S. H. W. & Hegde, R. S. Structure of the mammalian ribosome-Sec61 complex to 3.4 Å resolution. Cell 157, 1632–1643 (2014)
Shao, S., Brown, A., Santhanam, B. & Hegde, R. S. Structure and assembly pathway of the ribosome quality control complex. Mol. Cell 57, 433–444 (2015)
Pettersen, E. F. et al. UCSF Chimera–a visualization system for exploratory research and analysis. J. Comput. Chem. 25, 1605–1612 (2004)
Karcher, A., Schele, A. & Hopfner, K. P. X-ray structure of the complete ABC enzyme ABCE1 from Pyrococcus abyssi . J. Biol. Chem. 283, 7962–7971 (2008)
Brown, A. et al. Tools for macromolecular model building and refinement into electron cryo-microscopy reconstructions. Acta Crystallogr. D 71, 136–153 (2015)
Selmer, M. Structure of the 70S ribosome complexed with mRNA and tRNA. Science 313, 1935–1942 (2006)
Chan, P. P. & Lowe, T. M. GtRNAdb: a database of transfer RNA genes detected in genomic sequence. Nucleic Acids Res. 37, D93–D97 (2009)
Chen, V. B. et al. MolProbity: all-atom structure validation for macromolecular crystallography. Acta Crystallogr. D 66, 12–21 (2010)
Amunts, A. et al. Structure of the yeast mitochondrial large ribosomal subunit. Science 343, 1485–1489 (2014)
Acknowledgements
We thank C. Savva, F. de Haas, and S. Welsch for assisting with cryo-EM data collection, J. Grimmett and T. Darling for computing support, D. Barford for critically reading the manuscript, and I. Fernández, J. Llácer, G. Murshudov, S. Scheres, and R. Voorhees for useful discussions. Gctf is available on request from K. Zhang (kzhang@mrc-lmb.cam.ac.uk). This work was supported by the UK Medical Research Council (MC_UP_A022_1007 to R.S.H. and MC_U105184332 to V.R.). A.B. was supported by a Career Development Fellowship. S.S. was supported by a St John’s College Title A fellowship. J.M. thanks T. Dever, NICHD, and the NIH Oxford-Cambridge Scholars’ Program for support. V.R. was supported by a Wellcome Trust Senior Investigator award (WT096570), the Agouron Institute, and the Jeantet Foundation.
Author information
Authors and Affiliations
Contributions
A.B., S.S., R.S.H. and V.R. designed the study. S.S. purified complexes and prepared samples. A.B., S.S. and J.M. collected data. A.B. calculated the cryo-EM reconstructions, built the atomic models and interpreted the structure. A.B., S.S., R.S.H and V.R. wrote the manuscript. All authors discussed and commented on the final manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Extended data figures and tables
Extended Data Figure 1 eRF1(AAQ) stalls ribosomes at stop codons.
a, Line diagrams of mRNA encoding nascent chain (NC) substrates used in this study. The cytosolic portion of human Sec61β (residues 1–68, orange) was modified to contain an N-terminal 3 × Flag tag (green) for affinity purification and the autonomously-folding villin headpiece (VHP, blue) domain. The three stop codons were individually inserted after Val68 of Sec61β to generate substrates for eRF1(AAQ)-mediated stalling, or the mRNA was truncated after the same residue to generate an independently-stalling substrate. b, In vitro translation reactions of NC-stop substrates containing the indicated stop codon (see panel a) in the presence of [35S]methionine without or with excess eRF1 WT or eRF1(AAQ). Reactions were for 25 min at 32 °C and directly analyzed by SDS–PAGE and auto-radiography. The terminated (NC) and tRNA-associated (NC-tRNA) nascent chain products are indicated. Addition of eRF1(AAQ) selectively prevents peptide hydrolysis when the stop codon is reached. c, Anti-Flag affinity purifications of ribosome-nascent chains (RNCs) stalled either by mRNA truncation or at the UAA stop codon with eRF1(AAQ) (see a) were immunoblotted for the splitting factors Hbs1 and ABCE1. The different amounts of Hbs1 and ABCE1 co-purified despite identical nascent chain sequences in each RNC complex suggest that eRF1(AAQ) selectively traps ABCE1 on pre-termination complexes. d, SDS–PAGE and Coomassie staining of affinity-purified eRF1(AAQ)-stalled ribosome-nascent chains containing the UGA stop codon used for cryo-EM analysis. Bands corresponding to ribosomal proteins, ABCE1, and eRF1(AAQ), which were verified by immunoblotting and mass spectrometry (data not shown), are indicated.
Extended Data Figure 2 In silico 3D classification scheme for cryo-EM data sets.
Particles extracted from automated particle picking in RELION were subjected to 2D classification. Non-ribosomal particles were discarded and the remaining particles were combined for a 3D refinement. The resulting map was used as a reference for 3D classification, which typically isolated 5 distinct classes of ribosomal complexes with the indicated distributions. Classes containing 80S ribosomes with canonical P- and E-site tRNAs and weak factor density in the A-site (∼40%) were combined and subjected to another round of 3D classification for A-site occupancy. Approximately one-third of this population contained strong density for eRF1(AAQ) and ABCE1. These particles were combined for subsequent 3D refinement and movie processing. All four data sets (two for the UAA stop codon and one each for the UAG and UGA stop codons) were processed similarly. The eRF1(AAQ)–ABCE1-containing particles of the two UAA data sets after the two rounds of classification were combined for refinement to yield the final map.
Extended Data Figure 3 Quality of maps and models.
a, Fourier shell correlation (FSC) curves for the electron microscopy maps of each termination complex containing the indicated stop codon. b, Isolated eRF1(AAQ)–ABCE1 density from the UAA termination complex map coloured by local resolution. c, Fit of models to maps. FSC curves calculated between the refined model and the final map (black), and with the self- (blue) and cross-validated (magenta) correlations for each stop codon complex. The electron microscopy map of each termination complex coloured by local resolution (as in b) is displayed next to the corresponding curves.
Extended Data Figure 4 eRF1(AAQ) interactions within the termination complex.
a, Comparison of ribosome-bound eRF1(AAQ) (coloured by domain) with the crystal structure of eRF1 (PDB accession code 1DT9, grey) superposed on the C domain. Both the N and M domains of eRF1 rotate upon stop codon recognition on the ribosome. The P-site tRNA (green) and nascent chain (teal) are shown for orientation. b, Interaction of helix α2 of the N domain of eRF1(AAQ) (purple) with the anticodon stem loop (ASL) of the P-site tRNA (green). c, Superposition of the eRF1(AAQ) M domain (purple) with the eRF1 crystal structure (PDB accession code 1DT9) showing a 10 Å movement of the GGQ-loop to accommodate within the peptidyl transferase centre.
Extended Data Figure 5 Examples of map densities.
a, Density (from the UAG-containing termination complex) for the nascent chain (teal) attached to the CCA end of the P-site tRNA (green) is of sufficient resolution to model the defined sequence of the C-terminal end of the programmed nascent chain. This provides additional verification that the termination complexes are stalled at Val68 of Sec61β (human numbering) with the stop codon in the A-site (see also Extended Data Fig. 1a). A stacking interaction between an aromatic residue of the nascent chain and U4555 (blue) lining the ribosomal exit tunnel can also be observed. b, Densities for the interactions between the UAG stop codon (grey), a portion of h44 of 18S rRNA (yellow) and the YxCxxxF and NIKS motifs of eRF1(AAQ) (purple). The invariant isoleucine of the NIKS motif provides a hydrophobic base for the stacking of the +2 and +3 bases of the stop codon with A1825. Unlike the tyrosine and cysteine residues of the YxCxxxF motif, the phenylalanine does not contribute to stop codon recognition, but to the hydrophobic packing of the eRF1 N domain.
Extended Data Figure 6 Hydrogen bonds specify for uridine at the +1 position.
Chemical diagrams of uridine and cytidine with hydrogen bond donors (blue) and acceptors (magenta) indicated. Two of the three hydrogen bonds that uridine forms with Asn61 and Lys63 of the NIKS motif of eRF1(AAQ) (purple) are not possible with cytidine (see also Fig. 4a).
Rights and permissions
About this article
Cite this article
Brown, A., Shao, S., Murray, J. et al. Structural basis for stop codon recognition in eukaryotes. Nature 524, 493–496 (2015). https://doi.org/10.1038/nature14896
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nature14896
This article is cited by
-
The eRF1 degrader SRI-41315 acts as a molecular glue at the ribosomal decoding center
Nature Chemical Biology (2024)
-
tRNA therapeutics for genetic diseases
Nature Reviews Drug Discovery (2024)
-
A molecular glue degrader of eRF1 on the ribosome
Nature Chemical Biology (2024)
-
In silico prospecting of the mtDNA of Macrobrachium amazonicum from transcriptome data
BMC Genomics (2023)
-
Short tRNA anticodon stem and mutant eRF1 allow stop codon reassignment
Nature (2023)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.