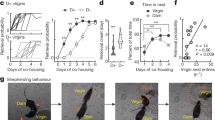
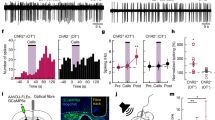
Abstract
Oxytocin is important for social interactions and maternal behaviour. However, little is known about when, where and how oxytocin modulates neural circuits to improve social cognition. Here we show how oxytocin enables pup retrieval behaviour in female mice by enhancing auditory cortical pup call responses. Retrieval behaviour required the left but not right auditory cortex, was accelerated by oxytocin in the left auditory cortex, and oxytocin receptors were preferentially expressed in the left auditory cortex. Neural responses to pup calls were lateralized, with co-tuned and temporally precise excitatory and inhibitory responses in the left cortex of maternal but not pup-naive adults. Finally, pairing calls with oxytocin enhanced responses by balancing the magnitude and timing of inhibition with excitation. Our results describe fundamental synaptic mechanisms by which oxytocin increases the salience of acoustic social stimuli. Furthermore, oxytocin-induced plasticity provides a biological basis for lateralization of auditory cortical processing.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Richard, P., Moos, F. & Freund-Mercier, M. J. Central effects of oxytocin. Physiol. Rev. 71, 331–370 (1991)
Gimpl, G. & Fahrenholz, F. The oxytocin receptor system: structure, function, and regulation. Physiol. Rev. 81, 629–683 (2001)
Insel, T. R. & Young, L. J. The neurobiology of attachment. Nature Rev. Neurosci. 2, 129–136 (2001)
Insel, T. R. The challenge of translation in social neuroscience: a review of oxytocin, vasopressin, and affiliative behavior. Neuron 65, 768–769 (2010)
Bartz, J. A., Zaki, J., Bolger, N. & Ochsner, K. N. Social effects of oxytocin in humans: Context and person matter. Trends Cogn. Sci. 15, 301–309 (2011)
Churchland, P. S. & Winkielman, P. Modulating social behavior with oxytocin: how does it work? What does it mean? Horm. Behav. 61, 392–399 (2012)
Pedersen, C. A., Ascher, J. A., Monroe, Y. L. & Prange, A. J. Oxytocin induces maternal behavior in virgin female rats. Science 216, 648–650 (1982)
Winslow, J. T. & Insel, T. R. Social status in pairs of male squirrel monkeys determines the behavioral response to central oxytocin administration. J. Neurosci. 11, 2032–2038 (1991)
Nishimori, K. et al. Oxytocin is required for nursing but is not essential for parturition or reproductive behavior. Proc. Natl Acad. Sci. USA 93, 11699–11704 (1996)
Zak, P. J., Stanton, A. A. & Ahmadi, S. Oxytocin increases generosity in humans. PLoS ONE 2, e1128 (2007)
Andari, E. et al. Promoting social behavior with oxytocin in high-functioning autism spectrum disorders. Proc. Natl Acad. Sci. USA 107, 4389–4394 (2010)
Chang, S. W. & Platt, M. L. Oxytocin and social cognition in rhesus macaques: Implications for understanding and treating human psychopathology. Brain Res. 1580, 57–68 (2014)
Dulac, C., O’Connell, L. A. & Wu, Z. Neural control of maternal and paternal behaviors. Science 345, 765–770 (2014)
Rilling, J. K. & Young, L. J. The biology of mammalian parenting and its effects on offspring social development. Science 345, 771–776 (2014)
Yoshida, M. et al. Evidence that oxytocin exerts anxiolytic effects via oxytocin receptor expressed in serotonergic neurons in mice. J. Neurosci. 29, 2259–2271 (2009)
Nakajima, M., Görlich, A. & Heintz, N. Oxytocin modulates female sociosexual behavior through a specific class of prefrontal cortical interneurons. Cell 159, 295–305 (2014)
Sewell, G. D. Ultrasonic communication in rodents. Nature 227, 410 (1970)
Noirot, E. Ultrasounds and maternal behavior in small rodents. Dev. Psychobiol. 5, 371–387 (1972)
Ehret, G. Left hemisphere advantage in the mouse brain for recognizing ultrasonic communication calls. Nature 325, 249–251 (1987)
Fichtel, I. & Ehret, G. Perception and recognition discriminated in the mouse auditory cortex by c-Fos staining. Neuroreport 10, 2341–2345 (1999)
Ehret, G. Infant rodent ultrasounds – a gate to the understanding of sound communication. Behav. Genet. 35, 19–29 (2005)
Crawley, J. N. Behavioral phenotyping strategies for mutant mice. Neuron 57, 809–818 (2008)
Cohen, L., Rothschild, G. & Mizrahi, A. Multisensory integration of natural odors and sounds in the auditory cortex. Neuron 72, 357–369 (2011)
Hofstetter, K. M. & Ehret, G. The auditory cortex of the mouse: connections of the ultrasonic field. J. Comp. Neurol. 323, 370–386 (1992)
Liu, R. C., Linden, J. F. & Schreiner, C. E. Improved cortical entrainment to infant communication calls in mothers compared with virgin mice. Eur. J. Neurosci. 23, 3087–3097 (2006)
Liu, R. C. & Schreiner, C. E. Auditory cortical detection and discrimination correlates with communicative significance. PLoS Biol. 5, e173 (2007)
Rothschild, G., Cohen, L., Mizrahi, A. & Nelken, I. Elevated correlations in neuronal ensembles of mouse auditory cortex following parturition. J. Neurosci. 33, 12851–12861 (2013)
Koch, M. & Ehret, G. Estradiol and parental experience, but not prolactin are necessary for ultrasound recognition and pup-retrieving in the mouse. Physiol. Behav. 45, 771–776 (1989)
Irani, B. G. et al. Distribution and neurochemical characterization of protein kinase C-theta and -delta in the rodent hypothalamus. Neuroscience 170, 1065–1079 (2010)
Wu, Z. et al. An obligate role of oxytocin neurons in diet induced energy expenditure. PLoS ONE 7, e45167 (2012)
Knobloch, H. S. et al. Evoked axonal oxytocin release in the central amygdala attenuates fear response. Neuron 73, 553–566 (2012)
Takayanagi, Y. et al. Pervasive social deficits, but normal parturition, in oxytocin receptor-deficient mice. Proc. Natl Acad. Sci. USA 102, 16096–16101 (2005)
Gong, S. et al. A gene expression atlas of the central nervous system based on bacterial artificial chromosomes. Nature 425, 917–925 (2003)
Wehr, M. & Zador, A. M. Balanced inhibition underlies tuning and sharpens spike timing in auditory cortex. Nature 426, 442–446 (2003)
Tan, A. Y. & Wehr, M. Balanced tone-evoked synaptic excitation and inhibition in mouse auditory cortex. Neuroscience 163, 1302–1315 (2009)
Dorrn, A. L., Yuan, K., Barker, A. J., Schreiner, C. E. & Froemke, R. C. Developmental sensory experience balances cortical excitation and inhibition. Nature 465, 932–936 (2010)
Froemke, R. C., Merzenich, M. M. & Schreiner, C. E. A synaptic memory trace for cortical receptive field plasticity. Nature 450, 425–429 (2007)
Kruglikov, I. & Rudy, B. Perisomatic GABA release and thalamocortical integration onto neocortical excitatory cells are regulated by neuromodulators. Neuron 58, 911–924 (2008)
Letzkus, J. J. et al. A disinhibitory microcircuit for associative fear learning in the auditory cortex. Nature 480, 331–335 (2011)
Froemke, R. C. et al. Long-term modification of cortical synapses improves sensory perception. Nature Neurosci. 16, 79–88 (2013)
Owen, S. F. et al. Oxytocin enhances hippocampal spike transmission by modulating fast-spiking interneurons. Nature 500, 458–462 (2013)
Rudick, C. N. & Woolley, C. S. Estrogen regulates functional inhibition of hippocampal CA1 pyramidal cells in the adult female rat. J. Neurosci. 21, 6532–6543 (2001)
Loring, D. W. et al. Cerebral language lateralization: evidence from intracarotid amobarbital testing. Neuropsychologia 28, 831–838 (1990)
Bishop, D. V. Cerebral asymmetry and language development: cause, correlate, or consequence? Science 340, 1230531 (2013)
Yoon, H., Enquist, L. W. & Dulac, C. Olfactory inputs to hypothalamic neurons controlling reproduction and fertility. Cell 123, 669–682 (2005)
Fraser, E. J. & Shah, N. M. Complex chemosensory control of female reproductive behaviors. PLoS ONE 9, e90368 (2014)
Wacker, D. W. & Ludwig, M. Vasopressin, oxytocin, and social odor recognition. Horm. Behav. 61, 259–265 (2012)
Lin, D. et al. Functional identification of an aggression locus in the mouse hypothalamus. Nature 470, 221–226 (2011)
Bennur, S., Tsunada, J., Cohen, Y. E. & Liu, R. C. Understanding the neurophysiological basis of auditory abilities for social communication: a perspective on the value of ethological paradigms. Hear. Res. 305, 3–9 (2013)
Wu, Z., Autry, A. E., Bergan, J. F., Watabe-Uchida, M. & Dulac, C. G. Galanin neurons in the medial preoptic area govern parental behavior. Nature 509, 325–330 (2014)
Huber, D., Veinante, P. & Stoop, R. Vasopressin and oxytocin excite distinct neuronal populations in the central amygdala. Science 308, 245–248 (2005)
Dölen, G., Darvishzadeh, A., Huang, K. W. & Malenka, R. C. Social reward requires coordinated activity of nucleus accumbens oxytocin and serotonin. Nature 501, 179–184 (2013)
Gunaydin, L. A. et al. Ultrafast optogenetic control. Nature Neurosci. 13, 387–392 (2010)
Kubota, Y. et al. Structure and expression of the mouse oxytocin receptor gene. Mol. Cell. Endocrinol. 124, 25–32 (1996)
Harris, J. A. et al. Anatomical characterization of Cre driver mice for neural circuit mapping and manipulation. Front. Neural Circuits 8, 76 (2014)
Fellous, J. M., Tiesinga, P. H., Thomas, P. J. & Sejnowski, T. J. Discovering spike patterns in neuronal responses. J. Neurosci. 24, 2989–3001 (2004)
Acknowledgements
We thank V. Azzara, R. Alicka, H. Bernstein, G. Buzsaki, I. Carcea, C. Grosso, M. Insanally, M. Jin, K. Kuchibhotla, B. Y. B. Lau, D. Lin, M. A. Long, N. Lopez, J. Marlin, C. McFarlane, E. Morina, S. Norden, D. Okobi, K. Peng, R. Priya, W. Rashid, L. Rubin, S. Shea, R. M. Sullivan, R. W. Tsien, D. Vallentin, L. J. Young and N. Zaika for comments, discussions and technical assistance, A. Mar and the NYU School of Medicine Behavioral Core for assistance with behavioural analysis, and C. A. Loomis and the NYU School of Medicine Histology Core for assistance with anatomical studies. Oxytocin-IRES-Cre mice were obtained from D. Olson and B. Lowell. Oxytocin receptor knockout mice were obtained from R. W. Tsien. Oxytocin receptor plasmid (pAAV-OXTR) was obtained from L. J. Young. Adeno-associated virus was obtained from the U. Penn Vector Core. S. E. Ross created artwork in Fig. 1a. This work was funded by NIDCD (DC009635, DC12557), a Klingenstein Fellowship, a McKnight Scholarship, a Pew Scholarship, a Sloan Research Fellowship, and a Whitehead Foundation Fellowship (R.C.F.); a Skirball Institute Collaborative Research Award (M.V.C. and R.C.F.); and NIMH (T32; B.J.M.).
Author information
Authors and Affiliations
Contributions
B.J.M. conducted behavioural studies and in vivo recordings. M.M. conducted anatomical studies. J.A.D. conducted in vitro recordings. M.M. and M.V.C. generated OXTR-2. R.C.F. and B.J.M. designed the study. All authors analysed data and wrote the paper.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Extended data figures and tables
Extended Data Figure 1 Expression of ChETA in oxytocinergic PVN neurons of Oxt-IRES-Cre animals.
a, Preparation of Oxt-IRES-Cre mice for optogenetic stimulation of endogenous oxytocin release. Animals had an adeno-associated virus (AAV) expressing the ChETA variant of channelrhodopsin-2 and YFP (pAAV5-EF1α-DIO-ChETA-eYFP) stereotaxically injected into the left PVN (left) and cannulas for fibre optic stimulation implanted either in the PVN (middle) or left AI (right). b, Confirmation of viral expression in PVN. Green, YFP; blue, DAPI. Scale bars, 100 μm.
Extended Data Figure 2 Retrieval success rates over time.
a, Examples of retrieval rate from three different virgin females receiving optogenetic stimulation of PVN (blue), oxytocin injections (red) or saline injections (black). Each data point is the average over 10 2-min trials immediately after injection or optical stimulation. b, Mean retrieval success at each time point for all co-housed virgin animals (including those that never retrieved within the first 3 days of co-housing). Some animals that began retrieving at earlier time points were not assessed at later time points and instead used for electrophysiological experiments.
Extended Data Figure 3 Oxytocin receptor expression in virgin female mouse auditory cortex, amygdala and lateral septum.
OXTR-2 labelling was detected in each area in wild-type but not oxytocin receptor knockout animals. a, Oxytocin receptor expression measured with OXTR-2 immunostaining in layer 5 of the left auditory cortex of naive wild-type animal (left) or oxytocin receptor knockout animal (right), imaged at ×20 magnification. Red, OXTR-2; blue, DAPI. b, OXTR-2 immunostaining in amygdala of wild-type animal (left) or oxytocin receptor knockout animal (right), imaged at ×10 magnification. c, OXTR-2 immunostaining in lateral septum of wild-type animal (left) or oxytocin receptor knockout animal (right), imaged at ×10 magnification. Scale bars, 100 μm.
Extended Data Figure 4 Oxytocinergic projections from hypothalamus to auditory cortex.
We identified YFP-positive axon segments in sections from Oxt-IRES-Cre animals. a, Oxytocinergic projections in left auditory cortex. Green, YFP; blue, DAPI. b, Oxytocinergic projections in right auditory cortex from same animal as in a. c, Oxytocinergic fibres and cells in PVN from same animal as in a and b. d, Quantification of axon segment length and number of axonal branches in left and right auditory cortex. No significant differences were found in total length or branch number in left vs. right cortex (left axon length: 0.94 ± 0.19 mm, right axon length: 0.92 ± 0.44 mm, n = 3 mice, P > 0.9, Student’s paired two-tailed t-test; left: 47.3 ± 6.0 mm axon branches, right: 45.6 ± 19.6 axon branches, P > 0.9). Scale bars, 100 μm.
Extended Data Figure 5 Spiking and synaptic pup call responses in dam right AI neurons.
a, Three example recordings from right AI neurons of experienced mothers. Left, current-clamp recording. Top, spectrogram of best pup call; middle, three example traces evoked by this call; bottom, raster plot showing spikes evoked over 12 trials (spiking z-score: 0.2; trial-by-trial average correlation r: 0.01). Middle, cell-attached recording (spiking z-score: 0.1; trial-by-trial correlation r: 0.01). Right, voltage-clamp recording. IPSCs and EPSCs shown were evoked by the best pup call (grey, individual trials; red, average; rei-best: 0.05, rei-all: −0.59). b, Summary of spiking responses in dam right (DR) AI neurons. Shown for comparison are responses from left AI neurons of dams (DL), naive virgins (NV), and experienced virgins (EV) from Fig. 4. Left, spiking responses to best pup calls (grey filled squares, current-clamp; open grey squares, cell-attached recordings; black squares, median z-score: 0.2 ± 0.8 (median ± s.e.m.), n = 7, P > 0.7 compared to naive virgin responses with Wilcoxon–Mann–Whitney two-sample rank test with Bonferroni correction for multiple comparisons, U = 76). Middle, spiking responses to pure tones (median z-score: 1.6 ± 0.3, n = 8, P > 0.4 compared to naive virgin responses, U = 83). Right, trial-by-trial correlation of pup call spiking responses (median r: 0.01 ± 0.04, n = 7, P > 0.6 compared to naive virgin responses, U = 78). Red squares indicate cells shown in a. c, Summary of synaptic responses in dam right AI neurons. Shown for comparison are responses from left AI neurons of dams, naive virgins, and experienced virgins from Fig. 5. Top left, EPSCs evoked by best pup calls (median EPSC: −11.0 ± 4.7, n = 4, P > 0.8 compared to naive virgin responses, U = 60). Bottom left, IPSCs evoked by best pup calls (median IPSC: 16.0 ± 7.0, n = 4, P > 0.4 compared to naive virgin responses, U = 68). Top middle, EPSCs evoked by pure tones (median EPSC: −33.8 ± 44.1, n = 7, P > 0.4 compared to naive virgin responses, U = 52). Bottom middle, IPSCs evoked by pure tones (median IPSC: 22.9 ± 35.7, n = 7, P > 0.7 compared to naive virgin responses, U = 45). Top right, excitatory–inhibitory correlations in temporal profiles of best call responses (median rei-best: 0.08 ± 0.14, n = 4, P > 0.7 compared to naive virgin responses, U = 60). Bottom right, excitatory–inhibitory correlations across all calls (median rei-all: 0.05 ± 0.37, n = 4, P > 0.8 compared to naive virgin responses, U = 59). Red triangles indicate cell shown in a. Error bars denote median ± interquartile range.
Extended Data Figure 6 Pup call responses in experienced versus naive females differ in timing but not overall amplitude.
a, Example periods of spontaneous excitation and inhibition in absence of pup call stimuli; same recordings as in Fig. 5a. b, Example call-evoked IPSCs and EPSCs from left AI neurons; same recordings as in Fig. 5a. Left column, neuron from dam. Top, spectrogram. Middle, IPSCs evoked by the best pup call (three individual trials are shown; average trial-by-trial correlation across all trials ri: 0.65). Note similarity and shared temporal structure across individual trials in this cell. Bottom, EPSCs evoked by this call (re: 0.85). Middle column, neuron from naive virgin (ri: −0.01; re: 0.02). Right column, neuron from experienced virgin (ri: 0.35; re: 0.55). c, Summary of spontaneous excitation (top, filled triangles) and inhibition (bottom, open triangles) measured as instantaneous current (pA). Spontaneous activity was similar in left AI neurons from dams (black triangle, median excitation: −1.2 ± 0.9 pA (median ± s.e.m.), n = 13, P > 0.7 compared to naive virgin spontaneous activity with Wilcoxon–Mann–Whitney two-sample rank test with Bonferroni correction for multiple comparisons, U = 178; open triangle, median inhibition: 0.6 ± 0.8 pA, P > 0.3 compared to naive virgin responses, U = 193), naive virgins (median excitation: −1.7 ± 0.7 pA, n = 28; median inhibition: 1.1 ± 0.8 pA), and experienced virgins (median excitation: −0.6 ± 0.7 pA, n = 13, P > 0.1 compared to naive virgin spontaneous activity, U = 232; median inhibition: 1.3 ± 0.7 pA, P > 0.5 compared to naive virgin responses, U = 168). Red triangles indicate cells shown in a and b. d, Summary of z-scored call-evoked EPSCs (top) and IPSCs (bottom) relative to spontaneous activity in dams (filled triangle, median excitation z-score: 1.8 ± 1.4, n = 13, P > 0.1 compared to naive virgin responses, U = 215; open triangle, median inhibition z-score: 2.0 ± 1.6, P > 0.1 compared to naive virgin responses, U = 181), naive virgins (median excitation z-score: 1.4 ± 0.3, n = 28; median inhibition z-score: 1.4 ± 0.6), and experienced virgins (median excitation z-score: 2.5 ± 1.0, n = 13, P > 0.1 compared to naive virgin responses, U = 250; median inhibition z-score: 2.7 ± 0.9, P < 0.02 compared to naive virgin responses, U = 228). Red triangles indicate cells shown in a and b. e, Summary of trial-by-trial correlations in temporal profile of best call responses for EPSCs (top, re) and IPSCs (bottom, ri) for dams (re: 0.25 ± 0.08, n = 12, P < 10−4 compared to naive virgin responses, U = 294; ri: 0.18 ± 0.08, P < 0.01 compared to naive virgin responses, U = 230), naive virgins (re: 0.0 ± 0.03, n = 28; ri: 0.01 ± 0.03), and experienced virgins (re: 0.31 ± 0.09, n = 13, P < 0.007 compared to naive virgin responses, U = 285; ri: 0.24 ± 0.07, P < 0.001 compared to naive virgin responses, U = 233). Red circles indicate cells shown in a and b. *P < 0.05, **P < 0.01. Error bars are median ± interquartile range.
Extended Data Figure 7 Simulations of spikes predicted from currents measured in voltage-clamp recordings.
a, Neuron from dam left AI (same cell as in Fig. 5a, left). Top, experimental data. Three representative EPSCs and three IPSCs evoked by the best call are displayed. Bottom, results of simulation. The membrane potential and spikes (clipped for display) of one trial run is shown, with a raster plot of 12 trials below. Yellow events indicate spikes that are synchronous within ∼10 ms on 50%+ trials. There was a high trial-to-trial correlation in spike firing (r: 0.26). b, Neuron from naive virgin left AI (same cell as in Fig. 5a, middle). Simulations using currents recorded in this cell predicted a low trial-to-trial correlation (r: 0.04). c, Neuron from experienced virgin left AI (same cell as in Fig. 5a, right). Simulations predicted a high trial-to-trial correlation (r: 0.31). d, Summary of simulated trial-by-trial spiking correlations for all voltage-clamp recordings from Fig. 5 of dams (r: 0.25 ± 0.04 (median ± s.e.m), n = 12, P < 0.0004 compared to simulated naive virgin responses with Wilcoxon–Mann–Whitney two-sample rank test with Bonferroni correction for multiple comparisons, U = 269), naive virgins (median r: 0.07 ± 0.02, n = 26), and experienced virgins (median r: 0.31 ± 0.10, n = 11, P < 0.04 compared to simulated naive virgin responses, U = 213). Red squares indicate cells shown in a–c. Note similarity to spike timing correlations measured experimentally and shown in Fig. 4e. **P < 0.01; *P < 0.05. e, Summary of simulated call-evoked firing rates of dams (median: 2.5 ± 1.1 Hz, n = 12, P > 0.8 compared to naive virgin responses, U = 161), naive virgins (median: 2.7 ± 0.5 Hz, n = 23), and experienced virgins (median: 3.2 ± 0.6 Hz, n = 11, P > 0.6 compared to naive virgin responses, U = 157). The simulated call-evoked responses were similar across each group due to the normalization of evoked current amplitudes; normalizing peak EPSCs and IPSCs allowed us to examine changes in spike timing independently from changes in overall spike rate. Error bars are median ± interquartile range.
Extended Data Figure 8 Oxytocin receptor activation disinhibits cortical neurons in brain slices.
a, Photomicrograph showing whole-cell recording from layer 5 pyramidal neuron in brain slice of virgin female mouse auditory cortex. b, Example voltage-clamp recording of IPSCs evoked by extracellular stimulation. Oxytocin was washed into the bath for 5 min. Red bar, duration of oxytocin wash-in. Dashed line, baseline IPSC amplitude. **P < 0.01. Inset, IPSCs before (black) and 3–5 min after wash-in (red). c, Example voltage-clamp recording of IPSCs evoked by extracellular stimulation in brain slice from Oxt-IRES-Cre mouse expressing ChETA in oxytocin neurons. Oxytocin release was evoked by blue light (hυ) for 3 min. *P < 0.05. d, Summary of changes to evoked IPSCs 3–5 min after oxytocin receptor activation, by exogenous oxytocin wash-in (red; 78.0 ± 5.3% (mean ± s.e.m.) of baseline amplitude; n = 12, P < 0.002 compared to baseline with Student’s two-tailed paired t-test; prevented by OTA, 108.6 ± 8.6% of baseline amplitude; n = 3, P > 0.4 compared to baseline) or by optogenetic release of endogenous oxytocin (86.7 ± 4.7% of baseline amplitude; n = 5, P < 0.05 compared to baseline; prevented by OTA, 101.2 ± 1.2% of baseline amplitude; n = 4, P > 0.8 compared to baseline). Error bars are mean ± s.e.m.
Extended Data Figure 9 Oxytocin pairing increases the trial-by-trial similarity of synaptic pup call responses.
a, Same voltage-clamp recording from virgin female left AI neuron as in Fig. 6c, showing that trial-by-trial similarity of call-evoked IPSCs and EPSCs is initially low but increases after oxytocin pairing. Shown are four representative IPSCs and EPSCs before pairing (inhibitory trial-by-trial correlation ri: 0.04, excitatory trial-by-trial correlation re: 0.00), during pairing (ri: 0.08, re: 0.49), 10–15 min after pairing (ri: −0.01, re: 0.58), and 45–50 min after pairing (ri: 0.13, re: 0.48). Scale bar, 75 pA (150 pA during pairing), 200 ms. b, Summary of changes to synaptic trial-by-trial correlations after oxytocin pairing in virgin left AI. Left, change in excitatory correlations (re) across multiple cells for hours after pairing. Blue, optogenetic pairing; red, oxytocin pairing; black, means binned over time (n = 28 cells from 17 animals; re before pairing: 0.01 ± 0.02 (mean ± s.e.m.), re 0–45 min after pairing: 0.16 ± 0.03, P < 0.0002 compared to values before pairing; re 1–3 h after pairing: 0.16 ± 0.04, P < 0.0009 compared to values before pairing). Dashed line, initial average re; arrow, time of pairing. Right, change in ri (ri before pairing: 0.01 ± 0.02, ri 0–45 min after pairing: 0.04 ± 0.02, P > 0.1 compared to values before pairing; ri 1–3 h after pairing: 0.14 ± 0.03, P < 0.0002 compared to values before pairing). Error bars are mean ± s.e.m.
Supplementary information
Pup retrieval by experienced mother
This video shows pup retrieval by an experienced mother. (WMV 3076 kb)
No retrieval by saline-injected virgin female
This video shows no retrieval by saline-injected virgin female. (WMV 1569 kb)
Pup retrieval by Oxt-IRES-Cre virgin female after PVN optical stimulation
This video shows pup retrieval by Oxt-IRES-Cre virgin female after PVN optical stimulation. (WMV 5148 kb)
Muscimol in left auditory cortex prevents pup retrieval
This video shows muscimol in left auditory cortex prevents pup retrieval. (WMV 6007 kb)
Pup retrieval by wild-type virgin female after left AI oxytocin infusion
This video shows pup retrieval by wild-type virgin female after left AI oxytocin infusion. (WMV 2777 kb)
Pup retrieval by Oxt-IRES-Cre virgin female after left AI optical stimulation
This video shows pup retrieval by Oxt-IRES-Cre virgin female after left AI optical stimulation. (WMV 5050 kb)
Source data
Rights and permissions
About this article
Cite this article
Marlin, B., Mitre, M., D’amour, J. et al. Oxytocin enables maternal behaviour by balancing cortical inhibition. Nature 520, 499–504 (2015). https://doi.org/10.1038/nature14402
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nature14402
This article is cited by
-
CD38 genetic variation is associated with increased personal distress to an emotional stimulus
Scientific Reports (2024)
-
Sensory cortex plasticity supports auditory social learning
Nature Communications (2023)
-
Distinct roles of amylin and oxytocin signaling in intrafamilial social behaviors at the medial preoptic area of common marmosets
Communications Biology (2023)
-
The neural circuit that makes maternal mice respond to pups’ cries
Nature (2023)
-
Brain-based gene expression of putative risk genes for anorexia nervosa
Molecular Psychiatry (2023)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.