Abstract
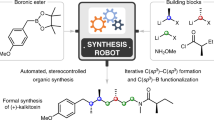
Molecular ‘assembly lines’, in which organic molecules undergo iterative processes such as chain elongation and functional group manipulation, are found in many natural systems, including polyketide biosynthesis. Here we report the creation of such an assembly line using the iterative, reagent-controlled homologation of a boronic ester. This process relies on the reactivity of α-lithioethyl tri-isopropylbenzoate, which inserts into carbon–boron bonds with exceptionally high fidelity and stereocontrol; each chain-extension step generates a new boronic ester, which is immediately ready for further homologation. We used this method to generate organic molecules that contain ten contiguous, stereochemically defined methyl groups. Several stereoisomers were synthesized and shown to adopt different shapes—helical or linear—depending on the stereochemistry of the methyl groups. This work should facilitate the rational design of molecules with predictable shapes, which could have an impact in areas of molecular sciences in which bespoke molecules are required.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
Accession codes
Data deposits
X-ray crystallographic data have been deposited in the Cambridge Crystallographic Data Centre database with accession numbers CCDC 993442 (12), CCDC 993443 (15) and CCDC 993441(17).
References
Staunton, J. & Weissman, K. J. Polyketide biosynthesis: a millennium review. Nat. Prod. Rep. 18, 380–416 (2001)
Hoffmann, R. W. Flexible molecules with defined shape: conformational design. Angew. Chem. Int. Edn Engl. 31, 1124–1134 (1992)
Hoffmann, R. W. Conformation design of open-chain compounds. Angew. Chem. Int. Edn Engl. 39, 2054–2070 (2000)
Smith, P. W. & Still, W. C. The effect of substitution and stereochemistry on ion binding in the polyether ionophore monensin. J. Am. Chem. Soc. 110, 7917–7919 (1988)
Wang, X., Erickson, S. D., Iimori, T. & Still, W. C. Enantioselective complexation of organic ammonium ions by simple tetracyclic podand ionophores. J. Am. Chem. Soc. 114, 4128–4137 (1992)
Wei, A., Boy, K. M. & Kishi, Y. Biological evaluation of rationally modified analogs of the H-type II blood group trisaccharide. A correlation between solution conformation and binding affinity. J. Am. Chem. Soc. 117, 9432–9436 (1995)
Boger, D. L., Ramsey, T. M., Cai, H., Hoehn, S. T. & Stubbe, J. Definition of the effect and role of the bleomycin A2 valerate substituents: preorganization of a rigid, compact conformation implicated in sequence-selective DNA cleavage. J. Am. Chem. Soc. 120, 9149–9158 (1998)
Nilewski, C., Geisser, R. W., Ebert, M.-O. & Carreira, E. M. Conformational and configurational analysis in the study and synthesis of chlorinated natural products. J. Am. Chem. Soc. 131, 15866–15876 (2009)
Hanessian, S., Giroux, S. & Mascitti, V. The iterative synthesis of acyclic deoxypropionate units and their implication in polyketide-derived natural products. Synthesis 7, 1057–1076 (2006)
ter Horst, B., Feringa, B. L. & Minnaard, A. J. Iterative strategies for the synthesis of deoxypropionates. Chem. Commun. 46, 2535–2547 (2010)
Dill, K. A. & MacCallum, J. L. The protein-folding problem, 50 years on. Science 338, 1042–1046 (2012)
Matteson, D. S. & Ray, R. Directed chiral synthesis with pinanediol boronic esters. J. Am. Chem. Soc. 102, 7590–7591 (1980)
Matteson, D. S. Boronic esters in asymmetric synthesis. J. Org. Chem. 78, 10009–10023 (2013)
Stymiest, J. L., Dutheuil, G., Mahmood, A. & Aggarwal, V. K. Lithiated carbamates: chiral carbenoids for iterative homologation of boranes and boronic esters. Angew. Chem. Int. Ed. 46, 7491–7494 (2007)
Besong, G., Jarowicki, K., Kocienski, P. J., Sliwinski, E. & Boyle, F. T. Synthesis of (S)-(−)-N-acetylcolchinol using intramolecular biaryl oxidative coupling. Org. Biomol. Chem. 4, 2193–2207 (2006)
Blakemore, P. R., Marsden, S. P. & Vater, H. D. Reagent controlled asymmetric homologation of boronic esters by enantioenriched main-group chiral carbenoids. Org. Lett. 8, 773–776 (2006)
Blakemore, P. R. & Burge, M. S. Iterative stereospecific reagent-controlled homologation of pinacol boronates by enantioenriched α-chloroalkyllithium reagents. J. Am. Chem. Soc. 129, 3068–3069 (2007)
Emerson, C. R., Zakharov, L. N. & Blakemore, P. R. Investigation of functionalized α-chloroalkyllithiums for a stereospecific reagent-controlled approach to the analgesic alkaloid (–)-epibatidine. Chemistry 19, 16342–16356 (2013)
Hoppe, D., Hintze, F. & Tebben, P. Chiral lithium-1-oxyalkanides by asymmetric deprotonation; enantioselective synthesis of 2-hydroxyalkanoic acids and secondary alkanols. Angew. Chem. Int. Edn Engl. 29, 1422–1424 (1990)
Hoppe, D. & Hense, T. Enantioselective synthesis with lithium/(−)-sparteine carbanion pairs. Angew. Chem. Int. Edn Engl. 36, 2282–2316 (1997)
Beckmann, E., Desai, V. & Hoppe, D. Stereospecific reaction of α-carbamoyloxy-2-alkenylboronates and α-carbamoyloxy-alkylboronates with Grignard reagents - synthesis of highly enantioenriched secondary alcohols. Synlett 13, 2275–2280 (2004)
Dutheuil, G., Webster, M. P., Worthington, P. A. & Aggarwal, V. K. Stereocontrolled synthesis of carbon chains bearing contiguous methyl groups by iterative boronic ester homologations: application to the total synthesis of (+)-faranal. Angew. Chem. Int. Ed. 48, 6317–6319 (2009)
Robinson, A. & Aggarwal, V. K. Asymmetric total synthesis of solandelactone E: stereocontrolled synthesis of the 1,4-diol-2-ene core via lithiation-borylation-allylation sequence. Angew. Chem. Int. Ed. 49, 6673–6675 (2010)
Pulis, A. P. & Aggarwal, V. K. Synthesis of enantioenriched tertiary boronic esters from secondary allylic carbamates. Application to the synthesis of C30 botryococcene. J. Am. Chem. Soc. 134, 7570–7574 (2012)
Fletcher, C. J., Wheelhouse, K. M. P. & Aggarwal, V. K. Stereoselective total synthesis of (+)-giganin and its C10 epimer by using late-stage lithiation–borylation methodology. Angew. Chem. Int. Ed. 52, 2503–2506 (2013)
Blair, D. J., Fletcher, C. J., Wheelhouse, K. M. P. & Aggarwal, V. K. Stereocontrolled synthesis of adjacent acyclic quaternary-tertiary motifs: application to a concise total synthesis of (–)-filiformin. Angew. Chem. Int. Ed. 53, 5552–5555 (2014)
Sun, X. & Blakemore, P. R. Programmed synthesis of a contiguous stereotriad motif by triple stereospecific reagent-controlled homologation. Org. Lett. 15, 4500–4503 (2013)
Larouche-Gauthier, R., Fletcher, C. J., Couto, I. & Aggarwal, V. K. Use of alkyl 2,4,6-triisopropylbenzoates in the asymmetric homologation of challenging boronic esters. Chem. Commun. (Camb.) 47, 12592–12594 (2011)
Still, W. C. & Sreekumar, C. α-Alkoxyorganolithium reagents. A new class of configurationally stable carbanions for organic synthesis. J. Am. Chem. Soc. 102, 1201–1202 (1980)
Rayner, P. J., O'Brien, P. & Horan, R. A. J. Preparation and reactions of enantiomerically pure α-functionalized Grignard reagents. J. Am. Chem. Soc. 135, 8071–8077 (2013)
Negishi, E. A quarter of a century of explorations in organozirconium chemistry. Dalton Trans. 827–848 (2005)
Vigneron, J. P., Dhaenens, M. & Horeau, A. Nouvelle methode pour porter au maximum la purete optique d’un produit partiellement dedouble sans l’aide d’aucune substance chirale. Tetrahedron 29, 1055–1059 (1973)
Tsuzuki, S. et al. Investigation of intramolecular interactions in n-alkanes. Cooperative energy increments associated with GG and GTG’ sequences. J. Am. Chem. Soc. 113, 4665–4671 (1991)
Lotz, B., Wittmann, J. C. & Lovinger, A. J. Structure and morphology of poly(propylenes): a molecular analysis. Polymer 37, 4979–4992 (1996)
Hunter, L., Kirsch, P., Slawin, A. M. Z. & O’Hagan, D. Synthesis and structure of stereoisomeric multivicinal hexafluoroalkanes. Angew. Chem. Int. Ed. 48, 5457–5460 (2009)
Hoffmann, R. W., Stahl, M., Schopfer, U. & Frenking, G. Conformation design of hydrocarbon backbones: a modular approach. Chemistry 4, 559–566 (1998)
Butts, C. P., Jones, C. R. & Harvey, J. N. High precision NOEs as a probe for low level conformers – a second conformation of strychnine. Chem. Commun. 47, 1193–1195 (2011)
Butts, C. P. et al. Interproton distance determinations by NOE – surprising accuracy and precision in a rigid organic molecule. Org. Biomol. Chem. 9, 177–184 (2011)
Adler, T. B., Werner, H. J. & Manby, F. R. Local explicitly correlated second-order perturbation theory for the accurate treatment of large molecules. J. Chem. Phys. 130, 054106 (2009)
Chini, M. G. et al. Quantitative ROE-derived interproton distances combined with quantum chemical calculations of NMR parameters in the stereochemical determination of conicasterol F, a nuclear receptor ligand from Theonella swinhoei. J. Org. Chem. 77, 1489–1496 (2012)
Di Micco, S., Chini, M. G., Riccio, R. & Bifulco, G. Quantum mechanical calculation of NMR parameters in the stereostructural determination of natural products. Eur. J. Org. Chem. 2010, 1411–1434 (2010)
Hanessian, S. et al. Application of conformation design in acyclic stereoselection: total synthesis of borrelidin as the crystalline benzene solvate. J. Am. Chem. Soc. 125, 13784–13792 (2003)
Brand, G. J., Studte, C. & Breit, B. Iterative synthesis of (oligo)deoxypropionates via zinc-catalyzed enantiospecific sp3−sp3 cross-coupling. Org. Lett. 11, 4668–4670 (2009)
ter Horst, B., Feringa, B. L. & Minnaard, A. J. Catalytic asymmetric synthesis of phthioceranic acid, a heptamethyl-branched acid from Mycobacterium tuberculosis. Org. Lett. 9, 3013–3015 (2007)
Han, S. B., Hassan, A., Kim, I. S. & Krische, M. J. Total synthesis of (+)-roxaticin via C−C bond forming transfer hydrogenation: a departure from stoichiometric chiral reagents, auxiliaries, and premetalated nucleophiles in polyketide construction. J. Am. Chem. Soc. 132, 15559–15561 (2010)
Lee, S. J., Gray, K. C., Paek, J. S. & Burke, M. D. Simple, efficient, and modular syntheses of polyene natural products via iterative cross-coupling. J. Am. Chem. Soc. 130, 466–468 (2008)
Wang, C. & Glorius, F. Controlled iterative cross-coupling: on the way to the automation of organic synthesis. Angew. Chem. Int. Ed. 48, 5240–5244 (2009)
Woerly, E. M., Roy, J. & Burke, M. D. Synthesis of most polyene natural product motifs using just 12 building blocks and one coupling reaction. Nat. Chem. 6, 484–491 (2014)
Negishi, E., Tan, Z., Liang, B. & Novak, T. An efficient and general route to reduced polypropionates via Zr-catalyzed asymmetric C—C bond formation. Proc. Natl Acad. Sci. USA 101, 5782–5787 (2004)
Albert, B. J. & Yamamoto, H. A triple-aldol cascade reaction for the rapid assembly of polyketides. Angew. Chem. Int. Ed. 49, 2747–2749 (2010)
Acknowledgements
We thank EPSRC (EP/I038071/1) and the European Research Council (FP7/2007-2013, ERC grant no. 246785) for financial support. M.B. thanks the EPSRC-funded Bristol Chemical Synthesis Centre for Doctoral Training (EP/G036764/1) and Novartis for a PhD studentship. We wish to thank C. Woodall for assistance with X-ray analysis and E. Bozoki for assistance with preparative high-performance liquid chromatography purification.
Author information
Authors and Affiliations
Contributions
V.K.A. designed the project. M.B. conducted and designed the experiments and analysed the data. S.E. performed computational studies and analysed the data with J.N.H. J.R.B. and S.P.B. performed the NMR experiments and analysed the data with C.P.B. M.P.W. conducted the preliminary experiments with lithiated carbamates. S.B. optimized the recrystallization conditions for stannane 5. J.W.D. supervised M.B. while working at Novartis. V.K.A., M.B., J.N.H. and C.P.B. wrote the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary information
This file contains Supplementary Text and Data – see Supplementary Contents page for details. (PDF 6277 kb)
Rights and permissions
About this article
Cite this article
Burns, M., Essafi, S., Bame, J. et al. Assembly-line synthesis of organic molecules with tailored shapes. Nature 513, 183–188 (2014). https://doi.org/10.1038/nature13711
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nature13711
This article is cited by
-
Stereoretentive enantioconvergent reactions
Nature Chemistry (2024)
-
Homologation reactions for olefin synthesis
Nature Synthesis (2024)
-
Photo-responsive functional materials based on light-driven molecular motors
Light: Science & Applications (2024)
-
Stepwise on-demand functionalization of multihydrosilanes enabled by a hydrogen-atom-transfer photocatalyst based on eosin Y
Nature Chemistry (2023)
-
Iterative synthesis of 1,3-polyboronic esters with high stereocontrol and application to the synthesis of bahamaolide A
Nature Chemistry (2023)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.