Abstract
The mechanisms by which mucosal homeostasis is maintained are of central importance to inflammatory bowel disease. Critical to these processes is the intestinal epithelial cell (IEC), which regulates immune responses at the interface between the commensal microbiota and the host1,2. CD1d presents self and microbial lipid antigens to natural killer T (NKT) cells, which are involved in the pathogenesis of colitis in animal models and human inflammatory bowel disease3,4,5,6,7,8. As CD1d crosslinking on model IECs results in the production of the important regulatory cytokine interleukin (IL)-10 (ref. 9), decreased epithelial CD1d expression—as observed in inflammatory bowel disease10,11—may contribute substantially to intestinal inflammation. Here we show in mice that whereas bone-marrow-derived CD1d signals contribute to NKT-cell-mediated intestinal inflammation, engagement of epithelial CD1d elicits protective effects through the activation of STAT3 and STAT3-dependent transcription of IL-10, heat shock protein 110 (HSP110; also known as HSP105), and CD1d itself. All of these epithelial elements are critically involved in controlling CD1d-mediated intestinal inflammation. This is demonstrated by severe NKT-cell-mediated colitis upon IEC-specific deletion of IL-10, CD1d, and its critical regulator microsomal triglyceride transfer protein (MTP)12,13, as well as deletion of HSP110 in the radioresistant compartment. Our studies thus uncover a novel pathway of IEC-dependent regulation of mucosal homeostasis and highlight a critical role of IL-10 in the intestinal epithelium, with broad implications for diseases such as inflammatory bowel disease.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Kaser, A., Zeissig, S. & Blumberg, R. S. Inflammatory bowel disease. Annu. Rev. Immunol. 28, 573–621 (2010)
Saenz, S. A., Taylor, B. C. & Artis, D. Welcome to the neighborhood: epithelial cell-derived cytokines license innate and adaptive immune responses at mucosal sites. Immunol. Rev. 226, 172–190 (2008)
Fuss, I. J. et al. Nonclassical CD1d-restricted NK T cells that produce IL-13 characterize an atypical Th2 response in ulcerative colitis. J. Clin. Invest. 113, 1490–1497 (2004)
Heller, F., Fuss, I. J., Nieuwenhuis, E. E., Blumberg, R. S. & Strober, W. Oxazolone colitis, a Th2 colitis model resembling ulcerative colitis, is mediated by IL-13-producing NK-T cells. Immunity 17, 629–638 (2002)
Olszak, T. et al. Microbial exposure during early life has persistent effects on natural killer T cell function. Science 336, 489–493 (2012)
Wingender, G. & Kronenberg, M. Role of NKT cells in the digestive system. IV. The role of canonical natural killer T cells in mucosal immunity and inflammation. Am. J. Physiol. Gastrointest. Liver Physiol. 294, G1–G8 (2008)
Heller, F. et al. Interleukin-13 is the key effector Th2 cytokine in ulcerative colitis that affects epithelial tight junctions, apoptosis, and cell restitution. Gastroenterology 129, 550–564 (2005)
Liao, C. M. et al. dysregulation of CD1d-restricted type II natural killer T cells leads to spontaneous development of colitis in mice. Gastroenterology 142, 326–334 (2012)
Colgan, S. P., Hershberg, R. M., Furuta, G. T. & Blumberg, R. S. Ligation of intestinal epithelial CD1d induces bioactive IL-10: critical role of the cytoplasmic tail in autocrine signaling. Proc. Natl Acad. Sci. USA 96, 13938–13943 (1999)
Perera, L. et al. Expression of nonclassical class I molecules by intestinal epithelial cells. Inflamm. Bowel Dis. 13, 298–307 (2007)
Blumberg, R. S. et al. Expression of a nonpolymorphic MHC class I-like molecule, CD1D, by human intestinal epithelial cells. J. Immunol. 147, 2518–2524 (1991)
Brozovic, S. et al. CD1d function is regulated by microsomal triglyceride transfer protein. Nature Med. 10, 535–539 (2004)
Zeissig, S. et al. Primary deficiency of microsomal triglyceride transfer protein in human abetalipoproteinemia is associated with loss of CD1 function. J. Clin. Invest. 120, 2889–2899 (2010)
El Marjou, F. et al. Tissue-specific and inducible Cre-mediated recombination in the gut epithelium. Genesis 39, 186–193 (2004)
Raabe, M. et al. Analysis of the role of microsomal triglyceride transfer protein in the liver of tissue-specific knockout mice. J. Clin. Invest. 103, 1287–1298 (1999)
Colgan, S. P. et al. Intestinal heat shock protein 110 regulates expression of CD1d on intestinal epithelial cells. J. Clin. Invest. 112, 745–754 (2003)
Chiu, Y. H. et al. Multiple defects in antigen presentation and T cell development by mice expressing cytoplasmic tail-truncated CD1d. Nature Immunol. 3, 55–60 (2002)
Saraiva, M. & O'Garra, A. The regulation of IL-10 production by immune cells. Nature Rev. Immunol. 10, 170–181 (2010)
Pickert, G. et al. STAT3 links IL-22 signaling in intestinal epithelial cells to mucosal wound healing. J. Exp. Med. 206, 1465–1472 (2009)
Yamagishi, N., Fujii, H., Saito, Y. & Hatayama, T. Hsp105β upregulates hsp70 gene expression through signal transducer and activator of transcription-3. FEBS J. 276, 5870–5880 (2009)
Chaudhry, A. et al. Interleukin-10 signaling in regulatory T cells is required for suppression of Th17 cell-mediated inflammation. Immunity 34, 566–578 (2011)
Rubtsov, Y. P. et al. Regulatory T cell-derived interleukin-10 limits inflammation at environmental interfaces. Immunity 28, 546–558 (2008)
Nakamura, J. et al. Targeted disruption of Hsp110/105 gene protects against ischemic stress. Stroke 39, 2853–2859 (2008)
Smiley, S. T., Kaplan, M. H. & Grusby, M. J. Immunoglobulin E production in the absence of interleukin-4-secreting CD1-dependent cells. Science 275, 977–979 (1997)
Kühn, R., Lohler, J., Rennick, D., Rajewsky, K. & Muller, W. Interleukin-10-deficient mice develop chronic enterocolitis. Cell 75, 263–274 (1993)
Roers, A. et al. T cell-specific inactivation of the interleukin 10 gene in mice results in enhanced T cell responses but normal innate responses to lipopolysaccharide or skin irritation. J. Exp. Med. 200, 1289–1297 (2004)
Mombaerts, P. et al. RAG-1-deficient mice have no mature B and T lymphocytes. Cell 68, 869–877 (1992)
Park, S. H., Roark, J. H. & Bendelac, A. Tissue-specific recognition of mouse CD1 molecules. J. Immunol. 160, 3128–3134 (1998)
Zeissig, S. et al. Hepatitis B virus-induced lipid alterations contribute to natural killer T cell-dependent protective immunity. Nature Med. 18, 1060–1068 (2012)
Vidal, K., Grosjean, I., Revillard, J.-P., Gespach, C. & Kaiserlian, D. Immortalization of mouse intestinal epithelial cells by the SV40-large T gene. Phenotypic and immune characterization of the MODE-K cell line. J. Immunol. Methods 166, 63–73 (1993)
Behar, S. M., Podrebarac, T. A., Roy, C. J., Wang, C. R. & Brenner, M. B. Diverse TCRs recognize murine CD1. J. Immunol. 162, 161–167 (1999)
Yue, S. C., Shaulov, A., Wang, R., Balk, S. P. & Exley, M. A. CD1d ligation on human monocytes directly signals rapid NF-κB activation and production of bioactive IL-12. Proc. Natl Acad. Sci. USA 102, 11811–11816 (2005)
Katayama, K. et al. A novel PPARγ gene therapy to control inflammation associated with inflammatory bowel disease in a murine model. Gastroenterology 124, 1315–1324 (2003)
Mizuguchi, H. & Kay, M. A. A simple method for constructing E1- and E1/E4-deleted recombinant adenoviral vectors. Hum. Gene Ther. 10, 2013–2017 (1999)
Chen, Z., Chen, L., Qiao, S. W., Nagaishi, T. & Blumberg, R. S. Carcinoembryonic antigen-related cell adhesion molecule 1 inhibits proximal TCR signaling by targeting ZAP-70. J. Immunol. 180, 6085–6093 (2008)
Kaser, A. et al. XBP1 links ER stress to intestinal inflammation and confers genetic risk for human inflammatory bowel disease. Cell 134, 743–756 (2008)
Furuta, G. T. et al. Eosinophils alter colonic epithelial barrier function: role for major basic protein. Am. J. Physiol. Gastrointest. Liver Physiol. 289, G890–G897 (2005)
Furuta, G. T. et al. Hypoxia-inducible factor 1-dependent induction of intestinal trefoil factor protects barrier function during hypoxia. J. Exp. Med. 193, 1027–1034 (2001)
Acknowledgements
The authors thank H.-C. Hung for technical assistance with microinjection, Y. Xie for performing osmium staining, A. Bedynek and M. Friedrich for performing immunohistochemistry of the human biopsies, F. A. Zhu for assistance with antigen presentation assays, D. Shouval, M. Sablon and D. Perez for animal care and husbandry, K. Tashiro for technical assistance with adenovirus preparation, V. M. Thiele for technical assistance, J. Cusick for help with manuscript preparation, and S. E. Plevy for discussions and reagents. This work was supported by: National Institutes of Health (NIH) (grants DK044319, DK051362, DK053056, DK088199) and the Harvard Digestive Diseases Center (DK0034854) (R.S.B.); the European Research Council (ERC Starting Grant agreement no. 336528), the Deutsche Forschungsgemeinschaft (DFG) (ZE 814/4-1, ZE 814/5-1, ZE 814/6-1), the Crohn’s and Colitis Foundation of America (Postdoctoral Fellowship Award), the European Commission (Marie Curie International Reintegration Grant no. 256363) and the DFG Excellence Cluster “Inflammation at Interfaces” (S.Z.); the DFG (OL 324/1-1) (T.O.); HL38180, DK56260, Washington University DDRCC P30DK52574 (morphology core) (N.O.D.); HDDC Pilot and Feasibility Grant (K.B.); NCI P30CA013696 (C.-S.L.), the DFG (BR 1912/6-1) and the Else Kroener-Fresenius-Stiftung (Else Kroener-Exzellenzstipendium 2010_EKES.32) (S.B.); Grant-in-Aid for Challenging Exploratory Research 24659823 from Japan Society for Promotion of Science (K.W.); the ERC under the European Community’s Seventh Framework Programme (FP7/2007-2013/ERC Grant agreement no. 260961), the National Institute for Health Research Cambridge Biomedical Research Centre, the Austrian Science Fund and Ministry of Science P21530-B18 and START Y446-B18, Innsbruck Medical University (MFI 2007-407) and the Addenbrooke’s Charitable Trust, CiCRA (A.K.); the European Community’s Seventh Framework Programme (FP7/2007-2013) under grant agreement SysmedIBD (no. 305564) (W.M., S.S.); the NIH (grants HL59561, DK034854, AI50950), the Helmsley Charitable Trust and the Wolpow Family Chair in IBD Treatment and Research (S.B.S.). PBS57-loaded and unloaded mouse CD1d tetramer was obtained through the NIH Tetramer Facility. The authors thank M. A. Exley and S. P. Colgan for discussions.
Author information
Authors and Affiliations
Contributions
T.O., J.F.N., C.M.D. and K.B. performed in vitro and in vivo experiments and analysed the results; N.O.D. performed osmium tetroxide staining; J.G. obtained and scored histopathologies; C.-S.L. generated Cd1d1fl/fl mice; C.J. contributed to the analysis of CD1dΔIEC mice; S.B. and K.S. contributed to the immunohistochemical analysis of ulcerative colitis patients; K.W., K. Katayama, A.N. and H.M. generated adenoviruses; K. Kawasaki and K.N. provided HSP110-KO mice; W.M. and S.B.S. provided and participated in the analysis of the Il10ΔIEC mice; S.S. contributed to the coordination of experimental studies; A.K. contributed to MttpΔIEC studies and to the analysis of microarray data; R.S.B. and S.Z. designed the study, coordinated the experimental work and wrote the manuscript with input from co-authors. All authors discussed the results and commented on the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Extended data figures and tables
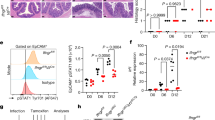
Extended Data Figure 1 Proposed model of CD1d signalling in polarized intestinal epithelia and overview of experimental procedures.
a, The proposed model of protective (blue) and pathogenic (red) effects of lipid antigen presentation in intestinal inflammation. Bone-marrow-derived antigen-presenting cells (APCs) contribute to oxazolone colitis in a CD1d- and iNKT-cell-dependent manner. By contrast, engagement of intestinal epithelial CD1d elicits protective functions through cytoplasmic CD1d tail-dependent activation of STAT3, and STAT3-dependent transcription of Cd1d1, Il10 and Hsph1. Epithelial IL-10 and HSP110 support this protective self-reinforcing pathway through STAT3-dependent signalling. Interference with any of the elements involved in this regulatory pathway (MTP, CD1d, IL-10 or HSP110) is associated with uncontrolled intestinal inflammation, thus highlighting a critical role of this pathway in the control of intestinal inflammation. Conv., conventional. b, Overview of experimental procedures.
Extended Data Figure 2 Absence of enterocyte lipid accumulation and ER stress in MttpΔIEC mice.
a, Absence of lipid accumulation in MttpΔIEC mice as shown in representative haematoxylin and eosin and rare osmium tetroxide (arrows) staining in the caecum, proximal, and distal colon. Scale bar, 25 µm. b, Xbp1 splicing in small intestinal (SI) and colonic IECs of the indicated mice before (left) (n = 5 mice per group) and 6 h after rectal challenge with oxazolone (Ox., right) (n = 5 mice per group). MODE-K cells were treated with either thapsigargin (Tg) or vehicle (DMSO) as positive and negative controls, respectively. Results representative of three independent experiments are shown.
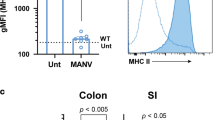
Extended Data Figure 3 Impaired CD1d- but not MHC-class I-restricted antigen presentation in MttpΔIEC mice.
a, CD1d-mediated presentation of the exogenous lipid antigen α-GalCer to the invariant NKT-cell hybridomas 24.7 and DN32.D3 and of endogenous lipid antigens (autoreactivity) to the non-invariant NKT-cell hybridoma 14S.6 by IECs from CD1d-knockout (CD1d-KO) mice, MttpΔIEC mice and wild-type littermates (Mttp+/+) (n = 5 mice per group). b, Presentation of H2-Kb-restricted SIINFEKL by the indicated IECs (see a) to the SIINFEKL-responsive hybridoma RF33.70. c, Representative histograms of CD1d cell surface expression as determined by flow cytometry of colonic IECs of the indicated mouse strains before (left, n = 5 mice per group) and 6 h after (right, n = 5 mice per group) rectal oxazolone challenge. d, Representative haematoxylin and eosin stainings of the indicated mouse strains upon rectal challenge with oxazolone (Ox.) or vehicle (ethanol (EtOH)). Scale bar, 40 μm. Results representative of three independent experiments are shown. Mean ± s.e.m. of triplicate cultures are shown. Student’s t-test was applied.
Extended Data Figure 4 Increased morbidity and mortality in oxazolone-challenged MttpΔIEC mice is due to CD1d-restricted components of the adaptive immune system.
a, Intestinal permeability as determined by FD-4 before and 18 h after rectal challenge with oxazolone (ox.) in the indicated mouse strains. Each symbol represents a single mouse. b, IL-1β secretion by CD11b+ cells from colonic lamina propria of the indicated bone marrow chimaeras. CD11b+ cells were isolated using magnetic microbeads 24 h after rectal challenge with oxazolone or ethanol and cultured for 24 h before measurement of IL-1β in culture supernatants by ELISA. Mean ± s.e.m. of triplicate cultures are shown. c, d, Survival and body weight of the indicated mouse strains at the indicated days after rectal oxazolone challenge. Results representative of three independent experiments are shown. Mean ± s.e.m. of the indicated number of mice are shown. Student’s t-test was applied.
Extended Data Figure 5 Development and characterization of mice with IEC-specific Cd1d1 deletion.
a, Schematic map of the targeting strategy for generation of Cd1d1fl/fl mice. A LoxP (L83) site was inserted at 10310 and a FNFL (Frt-Neo-Frt-LoxP) cassette at 12190 to flank exons 2, 3, and 4 (about 1.9 kb) of the Cd1d1 gene to generate the ‘floxed/neo’ Cd1d1 allele. A gene-targeting vector was constructed by retrieving one 5 kb long homology arm (5′ to L83), one 1.9 kb sequence containing exon 2/3/4, FNFL cassette, and one 2 kb short homology arm (end of FNFL to 3′). The FNFL cassette conferred G418 resistance during gene targeting in PTL1 (129B6 hybrid) embryonic stem (ES) cells. The targeted Cd1d1 allele was PCR amplified and sequenced to confirm the targeted C57BL/6 allele based on the C57BL/6-specific mutation in the Cd1d2 allele. Three targeted ES cells with targeted C57BL/6 allele were injected into C57BL/6 blastocysts to generate chimaeric mice. Male chimaeras were bred to bACTFlpe females or EIIa-Cre females to transmit the floxed Cd1d1 allele (Cd1d1L/+) (with neo cassette removed by Fple recombinase) through the germ line. Mice carrying the floxed Cd1d1 allele were crossed to tissue-specific VillinCre-ERT2 and exons 2, 3 and 4 and Frt-LoxP were removed by Cre recombinase in intestinal epithelial cells. b–e, Impaired CD1d- but not MHC-class I-restricted antigen presentation in Cd1d1ΔIEC mice. b, Cd1d1ΔIEC mice exhibit normal numbers of invariant NKT cells in liver, spleen and colonic lamina propria as determined by flow cytometry using α-GalCer/CD1d tetramers (n = 4 mice per group). c, Representative histograms of CD1d cell surface expression on colonic IECs of the indicated mouse strains before (left, n = 5 mice per group) and 6 h after (right, n = 5 mice per group) rectal oxazolone challenge. d, CD1d-mediated antigen presentation by colonic IECs of the indicated mouse strains (n = 5 mice per group). Presentation of the model glycolipid antigen α-GalCer to invariant NKT-cell hybridomas 24.7 and DN32.D3 and of endogenous antigens (autoreactivity) to the non-invariant NKT-cell hybridoma 14S.6 is shown. e, Presentation of H2-Kb-restricted SIINFEKL presentation by the indicated IECs (n = 5 mice per group) to the SIINFEKL-responsive hybridoma RF33.70. Results representative of three independent experiments are shown b–e, Mean ± s.e.m. of triplicate cultures are shown. Student’s t-test was applied. f, Intestinal epithelial CD1d is protective in oxazolone colitis. Representative macroscopic colon images of Cd1d1ΔIEC and wild-type littermates upon rectal challenge with oxazolone (Ox., n = 5 mice per group) or vehicle (ethanol (EtOH), n = 4 mice per group).
Extended Data Figure 6 Epithelial HSP110 expression is decreased in MttpΔIEC mice and in human ulcerative colitis.
a, HSP110 immunohistochemistry in mice with IEC Mttp deletion (bottom) as compared with wild-type littermates (top) 6 h after rectal oxazolone challenge. b, HSP110 immunohistochemistry in the colonic intestinal epithelium and the lamina propria of healthy controls and patients with active and inactive ulcerative colitis. Signal intensity was scored on a scale from 0 to 3 in a blinded fashion. Each symbol represents a single patient. The median and significance level as determined by the Mann–Whitney U-test are shown. c, Mortality in conventional HSP110-knockout mice in the oxazolone colitis model. Results representative of three independent experiments are shown. Mean ± s.e.m. of the indicated number of mice is shown. Student’s t-test and the log-rank test (survival) were applied. NS, not significant.
Extended Data Figure 7 Purity of isolated IECs.
After isolation of colonic IECs, potential contamination with haematopoetic cells was investigated by flow cytometry. The major population of cells was gated (upper left), was shown to contain viable cells (upper middle), and to contain largely EpCAM-positive epithelial cells that do not stain with leukocyte markers (middle and bottom). The upper right panel shows isotype control staining. Results representative of three independent experiments (n = 5 mice per experiment) are shown.
Extended Data Figure 8 CD1d-dependent STAT3 phosphorylation upon co-culture of IECs and iNKT cells.
a, STAT3 phosphorylation in IECs upon co-culture with iNKT cells is CD1d-dependent. Expression of pSTAT3, STAT3 and β-actin as determined by western blotting of MODE-K cells at the indicated time after addition of the iNKT-cell hybridoma 24.7. Where indicated, MODE-K cells were pre-incubated with a monoclonal blocking antibody directed against CD1d (19G11; +) or an isotype control (−). b, siRNA-mediated knockdown of Stat3 in MODE-K cells as determined 68 h after siRNA transfection. Results representative of three independent experiments are shown. Means ± s.e.m. of triplicates are shown. Student’s t-test was applied.
Extended Data Figure 9 CD1d-mediated cytokine production is dependent on the APC and expression of co-stimulatory molecules.
a–f, IL-10 (a, c–f) and IL-12p70 (b) secretion of co-cultures of the IEC line MODE-K or splenic dendritic cells (DCs) together with the iNKT-cell hybridoma 24.7. APCs were loaded with α-GalCer (αGC; 100 ng ml−1) before washing and co-culture with iNKT cells. c, MODE-K cells (IEC) were transfected with control siRNA or siRNA directed against Il10 before co-culture with iNKT cells. d, f, MODE-K cells were transfected with CD40 (d) and CD1d (f) as indicated. Histograms demonstrated increased expression of CD40 (d) and CD1d (f) after transfection. e, Stimulatory anti-CD28 antibody was added during co-culture of MODE-K and iNKT cells. Means ± s.e.m. of quadruplicate cultures are shown. Student’s t-test was applied. Results are representative of two independent experiments.
Supplementary information
Supplementary Information
This file contains a Supplementary Discussion of potential mechanisms responsible for divergent outcomes of CD1d engagement on intestinal epithelial cells and professional antigen presenting cells. (PDF 126 kb)
Rights and permissions
About this article
Cite this article
Olszak, T., Neves, J., Dowds, C. et al. Protective mucosal immunity mediated by epithelial CD1d and IL-10. Nature 509, 497–502 (2014). https://doi.org/10.1038/nature13150
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nature13150
This article is cited by
-
CD1-mediated immune responses in mucosal tissues: molecular mechanisms underlying lipid antigen presentation system
Experimental & Molecular Medicine (2023)
-
The microbiota and the gut–liver axis in primary sclerosing cholangitis
Nature Reviews Gastroenterology & Hepatology (2023)
-
CD1d-dependent rewiring of lipid metabolism in macrophages regulates innate immune responses
Nature Communications (2022)
-
Evaluation of the Function of Probiotics, Emphasizing the Role of their Binding to the Intestinal Epithelium in the Stability and their Effects on the Immune System
Biological Procedures Online (2021)
-
Host immunomodulatory lipids created by symbionts from dietary amino acids
Nature (2021)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.