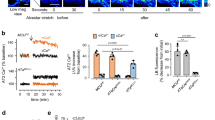
Abstract
The tissue-resident macrophages of barrier organs constitute the first line of defence against pathogens at the systemic interface with the ambient environment. In the lung, resident alveolar macrophages (AMs) provide a sentinel function against inhaled pathogens1. Bacterial constituents ligate Toll-like receptors (TLRs) on AMs2, causing AMs to secrete proinflammatory cytokines3 that activate alveolar epithelial receptors4, leading to recruitment of neutrophils that engulf pathogens5,6. Because the AM-induced response could itself cause tissue injury, it is unclear how AMs modulate the response to prevent injury. Here, using real-time alveolar imaging in situ, we show that a subset of AMs attached to the alveolar wall form connexin 43 (Cx43)-containing gap junction channels with the epithelium. During lipopolysaccharide-induced inflammation, the AMs remained sessile and attached to the alveoli, and they established intercommunication through synchronized Ca2+ waves, using the epithelium as the conducting pathway. The intercommunication was immunosuppressive, involving Ca2+-dependent activation of Akt, because AM-specific knockout of Cx43 enhanced alveolar neutrophil recruitment and secretion of proinflammatory cytokines in the bronchoalveolar lavage. A picture emerges of a novel immunomodulatory process in which a subset of alveolus-attached AMs intercommunicates immunosuppressive signals to reduce endotoxin-induced lung inflammation.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Laskin, D. L., Sunil, V. R., Gardner, C. R. & Laskin, J. D. Macrophages and tissue injury: agents of defense or destruction? Annu. Rev. Pharmacol. Toxicol. 51, 267–288 (2011)
Medzhitov, R. Toll-like receptors and innate immunity. Nature Rev. Immunol. 1, 135–145 (2001)
Thorley, A. J. et al. Differential regulation of cytokine release and leukocyte migration by lipopolysaccharide-stimulated primary human lung alveolar type II epithelial cells and macrophages. J. Immunol. 178, 463–473 (2007)
Kuebler, W. M., Parthasarathi, K., Wang, P. M. & Bhattacharya, J. A novel signaling mechanism between gas and blood compartments of the lung. J. Clin. Invest. 105, 905–913 (2000)
Maus, U. A. et al. Role of resident alveolar macrophages in leukocyte traffic into the alveolar air space of intact mice. Am. J. Physiol. Lung Cell. Mol. Physiol. 282, L1245–L1252 (2002)
Zhang, P., Summer, W. R., Bagby, G. J. & Nelson, S. Innate immunity and pulmonary host defense. Immunol. Rev. 173, 39–51 (2000)
Cohen, T. S. & Prince, A. S. Activation of inflammasome signaling mediates pathology of acute P. aeruginosa pneumonia. J. Clin. Invest. 123, 1630–1637 (2013)
Guth, A. M. et al. Lung environment determines unique phenotype of alveolar macrophages. Am. J. Physiol. Lung Cell. Mol. Physiol. 296, L936–L946 (2009)
Caton, M. L., Smith-Raska, M. R. & Reizis, B. Notch–RBP-J signaling controls the homeostasis of CD8− dendritic cells in the spleen. J. Exp. Med. 204, 1653–1664 (2007)
Srinivas, S. et al. Cre reporter strains produced by targeted insertion of EYFP and ECFP into the ROSA26 locus. BMC Dev. Biol. 1, 4 (2001)
Islam, M. N. et al. Mitochondrial transfer from bone-marrow-derived stromal cells to pulmonary alveoli protects against acute lung injury. Nature Med. 18, 759–765 (2012)
Miller, J. C. et al. Deciphering the transcriptional network of the dendritic cell lineage. Nature Immunol. 13, 888–899 (2012)
Thornton, E. E. et al. Spatiotemporally separated antigen uptake by alveolar dendritic cells and airway presentation to T cells in the lung. J. Exp. Med. 209, 1183–1199 (2012)
Kirby, A. C., Coles, M. C. & Kaye, P. M. Alveolar macrophages transport pathogens to lung draining lymph nodes. J. Immunol. 183, 1983–1989 (2009)
Lindert, J., Perlman, C. E., Parthasarathi, K. & Bhattacharya, J. Chloride-dependent secretion of alveolar wall liquid determined by optical-sectioning microscopy. Am. J. Respir. Cell Mol. Biol. 36, 688–696 (2007)
Pfenniger, A., Chanson, M. & Kwak, B. R. Connexins in atherosclerosis. Biochim. Biophys. Acta 1828, 157–166 (2013)
Ichimura, H., Parthasarathi, K., Lindert, J. & Bhattacharya, J. Lung surfactant secretion by interalveolar Ca2+ signaling. Am. J. Physiol. Lung Cell. Mol. Physiol. 291, L596–L601 (2006)
Wong, C. W. et al. Connexin37 protects against atherosclerosis by regulating monocyte adhesion. Nature Med. 12, 950–954 (2006)
Akira, S. & Takeda, K. Toll-like receptor signalling. Nature Rev. Immunol. 4, 499–511 (2004)
Li, H. et al. Toll-like receptor 4-myeloid differentiation factor 88 signaling contributes to ventilator-induced lung injury in mice. Anesthesiology 113, 619–629 (2010)
Imai, Y. et al. Identification of oxidative stress and Toll-like receptor 4 signaling as a key pathway of acute lung injury. Cell 133, 235–249 (2008)
Subramanian, M., Thorp, E., Hansson, G. K. & Tabas, I. Treg-mediated suppression of atherosclerosis requires MYD88 signaling in DCs. J. Clin. Invest. 123, 179–188 (2013)
Yano, S., Tokumitsu, H. & Soderling, T. R. Calcium promotes cell survival through CaM-K kinase activation of the protein-kinase-B pathway. Nature 396, 584–587 (1998)
Chen, B. C., Wu, W. T., Ho, F. M. & Lin, W. W. Inhibition of interleukin-1β-induced NF-κB activation by calcium/calmodulin-dependent protein kinase kinase occurs through Akt activation associated with interleukin-1 receptor-associated kinase phosphorylation and uncoupling of MyD88. J. Biol. Chem. 277, 24169–24179 (2002)
Perl, A. K., Wert, S. E., Nagy, A., Lobe, C. G. & Whitsett, J. A. Early restriction of peripheral and proximal cell lineages during formation of the lung. Proc. Natl Acad. Sci. USA 99, 10482–10487 (2002)
Parthasarathi, K. et al. Connexin 43 mediates spread of Ca2+-dependent proinflammatory responses in lung capillaries. J. Clin. Invest. 116, 2193–2200 (2006)
Wang, L. et al. Hypoxic pulmonary vasoconstriction requires connexin 40-mediated endothelial signal conduction. J. Clin. Invest. 122, 4218–4230 (2012)
Decrock, E. et al. IP3, a small molecule with a powerful message. Biochim. Biophys. Acta 1833, 1772–1786 (2013)
Huang, B. X. & Kim, H. Y. Effective identification of Akt interacting proteins by two-step chemical crosslinking, co-immunoprecipitation and mass spectrometry. PLoS ONE 8, e61430 (2013)
Centers for Disease Conrol and Prevention. Methicillin-resistant Staphylococcus aureus infections in correctional facilities—Georgia, California, and Texas, 2001–2003. MMWR Morb. Mortal. Wkly. Rep. 52, 992–996 (2003)
Rowlands, D. J. et al. Activation of TNFR1 ectodomain shedding by mitochondrial Ca2+determines the severity of inflammation in mouse lung microvessels. J. Clin. Invest. 121, 1986–1999 (2011)
Acknowledgements
We thank B. Reizis for providing the Cd11c-cre mice and J. Whitsett for providing the Spc-cre mice. We thank I. Tabas for discussions. This study was supported by US National Institutes of Health grants HL78645, HL57556 and HL64896 to J.B., HL73989 to A.S.P., and Parker B. Francis Fellowships to T.S.C. and M.N.I.
Author information
Authors and Affiliations
Contributions
K.W. designed and carried out the experiments, prepared the figures and wrote the initial manuscript. G.A.G. contributed to western blot, immunoprecipitation and FRAP experiments. M.N.I. carried out the NF-κB in situ stainings, and contributed to BAL cell counting and survival studies. M.S. performed the antigen-presentation assay and provided the CD11c Myd88−/− mice. T.S.C. provided S. aureus and contributed to ELISA studies. A.S.P. contributed to the experimental design. J.B. was responsible for the overall project, designed the experiments and wrote the initial manuscript. All authors edited the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Extended data figures and tables
Extended Data Figure 1 AM function and distribution
a, Fluorescence-activated cell sorting (FACS) plots and group data for antigen-presentation assays on AMs from BAL samples. First box shows gating scheme for CD11chigh MHC-IIlow AMs (black box). n = 3; −, negative control; +, positive control. *P < 0.05 versus negative control. b, Quantifications of AMs and alveoli (Alv). *P < 0.05 versus left bar; n = 20 imaging fields. c, Images and quantifications of AM numbers before (top) and after (bottom) indicated treatments (n = 4). Arrowheads indicate AMs. 4 h, 4 h imaging; BAL, 3× 1 ml BAL; Bu, 20 min injection of buffer containing calcein; SA, S. aureus injection. Scale bar, 30 μm. *P < 0.05 versus before. d, Gating scheme for sorting of CD11chigh MHC-IIlow AMs (black box) from lung tissue samples. Bars are mean ± s.e.m.
Extended Data Figure 2 Cx43 knockout in AMs.
a, Images show CD11c (green) and Cx43 (red) immunofluorescence and nuclei (blue) in lung-tissue-adherent AMs of wild-type (top) and CD11c Cx43−/− (Cx−/−; middle) mice. Bottom image is IgG control for Cx43 antibody. Bars quantify Cx43 expression. Ctl, control. Scale bar, 5 μm. *P < 0.05 versus control; n = 4. b, Sequential images show progressive colocalization (yellow) of AMs (arrowheads) and S. aureus (green). Scale bar, 15 μm. Replicated three times. c, Bars quantify AM numbers 1 h after bacterial challenge. n = 4 for wild type, n = 3 for CD11c Cx43−/−. Bars are mean ± s.e.m.
Extended Data Figure 3 Spatiotemporal uptake of fluorescent LPS in AMs and lung dendritic cells.
a, Gating scheme for sorting of CD11chigh MHC-IIlow AMs and CD11chigh MHC-IIlhigh dendritic cells (DCs; black boxes) from BAL (top) and lung tissue (bottom) samples. b, Representative FACS plots for fluorescent LPS uptake (green box) in AMs (black line) and dendritic cells (red line) recovered from BAL (left) and lung tissue samples after BAL (right). c, Group data quantify percentages of dendritic cells and AMs among CD11c-expressing cells in BAL (B) and lung tissue after BAL (T). d, Quantification of LPS uptake in cells of AM and dendritic-cell populations shown in c. n = 4 for tissue, n = 5 for BAL samples. Bars are mean ± s.e.m. *P < 0.05.
Extended Data Figure 4 AM distribution and intercommunication of Ca2+ spikes.
a, Images and sketch show the alveolar trajectory (dashed line) of a Ca2+ spike that originates in one AM (left arrowhead) and passes through alveolar type II cells (arrows) to another AM (right arrowhead). Scale bar, 20 µm. b, c, Bars quantify fluo-4 fluorescence in baseline (b) and 24 h LPS-treated (c) lungs at the beginning (0) and after 20 min of imaging (20) (n = 4). d, Quantification of AM Ca2+ spikes 4 h after LPS in control (Ctl, n = 4) and leukocyte-depleted (Leu, n = 3) lungs. e, Bar diagram shows AMs per imaging field in untreated lungs (Ctl) and at different time points after LPS. Ctl, n = 8; 1 h, n = 6; 4 h, n = 4; 24 h, n = 11. f, Bars quantify Cx43 expression. My−/−, CD11c Myd88−/−. Bars are mean ± s.e.m. n = 4.
Extended Data Figure 5 Cellular responses to E. coli.
a, Bars quantify BAL leukocytes 24 h after E. coli instillation in littermate control (Ctl) and CD11c Cx43−/− (Cx−/−) mice. Bars are mean ± s.e.m. *P < 0.05 versus control, n = 8. b, Western blots for phosphorylated (p-Akt) and total Akt in whole lung homogenates (n = 4 lungs).
Extended Data Figure 6 Cytokine secretion in response to Cx43 knockdown in bone-marrow-derived macrophages.
Cells were treated with scrambled or siRNA against Cx43. Differences were significant between LPS and PBS (P < 001), but not within groups (n = 4). Bars are mean ± s.e.m. ND, not detected.
Extended Data Figure 7 NF-κB immunostaining in the alveolar epithelium and AM distribution in knockout mice.
a, Images show immunofluorescence for NF-κB (green) and alveolar nuclei (red) for CD11c Cx43−/− mice (Cx−/−) and littermate controls (Ctl) (n = 4). Rectangle in merged images depicts magnified nuclei. b, Bars quantify AMs per imaging field in optically viewed lungs 24 h after LPS instillation. Ctl, wild-type mice (n = 11). Cx−/−, CD11c Cx43−/− mice (n = 6); My−/−, CD11c Myd88−/− mice (n = 6). Scale bar, 10 μm. Bars are mean ± s.e.m.
Extended Data Figure 8 Ca2+ dependence of Akt phosphorylation and BAL leukocyte counts and p-Akt expression in SPC Cx43−/− mice.
a, Western blots show phosphorylated Akt (p-Akt) and total Akt after instillations of LPS or LPS plus BAPTA (100 µM) 4 h before lung isolation (n = 4). b, Bars quantify BAL leukocytes 24 h after LPS instillation in littermate control (Ctl) and SPC Cx43−/− (SCx−/−) mice. Bars are mean ± s.e.m. *P < 0.05 versus control, n = 4. Gels are representative western blots for phosphorylated Akt, Cx43 and total Akt in whole lung homogenates (n = 4).
Supplementary information
Supplementary Figures
This file contains Supplementary Figure 1, which contains the alveolar markings for Supplementary Video 1. (PDF 201 kb)
Leukocyte movement in the alveolar airspace.
This movie file contains a 10-min imaging sequence from alveoli shown in Supplementary Figure 1. It depicts intra-alveolar leukocyte motility after LPS challenge. (MOV 22339 kb)
Intercommunication of AM Ca2+ spikes.
This file contains shows additional information for figure 2b in the main paper. The movie montage shows Ca2+ spikes (fluorescence increases) in AMs that travel from left to right. (MOV 3389 kb)
Source data
Rights and permissions
About this article
Cite this article
Westphalen, K., Gusarova, G., Islam, M. et al. Sessile alveolar macrophages communicate with alveolar epithelium to modulate immunity. Nature 506, 503–506 (2014). https://doi.org/10.1038/nature12902
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nature12902
This article is cited by
-
Physiological and immunological barriers in the lung
Seminars in Immunopathology (2024)
-
Innate immune responses in pneumonia
Pneumonia (2023)
-
B cell-derived IL-10 promotes the resolution of lipopolysaccharide-induced acute lung injury
Cell Death & Disease (2023)
-
Metabolic reprogramming of proinflammatory macrophages by target delivered roburic acid effectively ameliorates rheumatoid arthritis symptoms
Signal Transduction and Targeted Therapy (2023)
-
Crystal ribcage: a platform for probing real-time lung function at cellular resolution
Nature Methods (2023)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.