Abstract
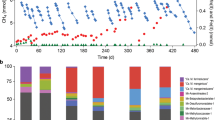
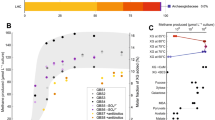
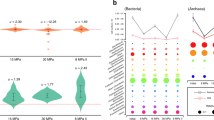
Anaerobic oxidation of methane (AOM) is critical for controlling the flux of methane from anoxic environments. AOM coupled to iron1, manganese1 and sulphate2 reduction have been demonstrated in consortia containing anaerobic methanotrophic (ANME) archaea. More recently it has been shown that the bacterium Candidatus ‘Methylomirabilis oxyfera’ can couple AOM to nitrite reduction through an intra-aerobic methane oxidation pathway3. Bioreactors capable of AOM coupled to denitrification have resulted in the enrichment of ‘M. oxyfera’ and a novel ANME lineage, ANME-2d4,5. However, as ‘M. oxyfera’ can independently couple AOM to denitrification, the role of ANME-2d in the process is unresolved. Here, a bioreactor fed with nitrate, ammonium and methane was dominated by a single ANME-2d population performing nitrate-driven AOM. Metagenomic, single-cell genomic and metatranscriptomic analyses combined with bioreactor performance and 13C- and 15N-labelling experiments show that ANME-2d is capable of independent AOM through reverse methanogenesis using nitrate as the terminal electron acceptor. Comparative analyses reveal that the genes for nitrate reduction were transferred laterally from a bacterial donor, suggesting selection for this novel process within ANME-2d. Nitrite produced by ANME-2d is reduced to dinitrogen gas through a syntrophic relationship with an anaerobic ammonium-oxidizing bacterium, effectively outcompeting ‘M. oxyfera’ in the system. We propose the name Candidatus ‘Methanoperedens nitroreducens’ for the ANME-2d population and the family Candidatus ‘Methanoperedenaceae’ for the ANME-2d lineage. We predict that ‘M. nitroreducens’ and other members of the ‘Methanoperedenaceae’ have an important role in linking the global carbon and nitrogen cycles in anoxic environments.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Beal, E. J., House, C. H. & Orphan, V. J. Manganese- and Iron-Dependent Marine Methane Oxidation. Science 325, 184–187 (2009)
Boetius, A. et al. A marine microbial consortium apparently mediating anaerobic oxidation of methane. Nature 407, 623–626 (2000)
Ettwig, K. F. et al. Nitrite-driven anaerobic methane oxidation by oxygenic bacteria. Nature 464, 543–548 (2010)
Raghoebarsing, A. A. et al. A microbial consortium couples anaerobic methane oxidation to denitrification. Nature 440, 918–921 (2006)
Hu, S. et al. Enrichment of denitrifying anaerobic methane oxidizing microorganisms. Environmental Microbiol. Rep. 1, 377–384 (2009)
Knittel, K. & Boetius, A. Anaerobic oxidation of methane: progress with an unknown process. Annu. Rev. Microbiol. 63, 311–334 (2009)
Reeburgh, W. S. Oceanic Methane Biogeochemistry. Chem. Rev. 107, 486–513 (2007)
Hallam, S. J., Girguis, P. R., Preston, C. M., Richardson, P. M. & DeLong, E. F. Identification of methyl coenzyme M reductase A (mcrA) genes associated with methane-oxidizing archaea. Appl. Environ. Microbiol. 69, 5483–5491 (2003)
Meyerdierks, A. et al. Metagenome and mRNA expression analyses of anaerobic methanotrophic archaea of the ANME-1 group. Environ. Microbiol. 12, 422–439 (2010)
Stokke, R., Roalkvam, I., Lanzen, A., Haflidason, H. & Steen, I. H. Integrated metagenomic and metaproteomic analyses of an ANME-1-dominated community in marine cold seep sediments. Environ. Microbiol. 14, 1333–1346 (2012)
Heller, C., Hoppert, M. & Reitner, J. Immunological localization of coenzyme M reductase in anaerobic methane-oxidizing archaea of ANME 1 and ANME 2 type. Geomicrobiol. J. 25, 149–156 (2008)
Scheller, S., Goenrich, M., Boecher, R., Thauer, R. K. & Jaun, B. The key nickel enzyme of methanogenesis catalyses the anaerobic oxidation of methane. Nature 465, 606–608 (2010)
Milucka, J. et al. Zero-valent sulphur is a key intermediate in marine methane oxidation. Nature 491, 541–546 (2012)
Hu, S., Zeng, R. J., Keller, J., Lant, P. A. & Yuan, Z. Effect of nitrate and nitrite on the selection of microorganisms in the denitrifying anaerobic methane oxidation process. Environmental Microbiol. Rep. 3, 315–319 (2011)
Strous, M. et al. Deciphering the evolution and metabolism of an anammox bacterium from a community genome. Nature 440, 790–794 (2006)
Yilmaz, S., Haroon, M. F., Rabkin, B. A., Tyson, G. W. & Hugenholtz, P. Fixation-free fluorescence in situ hybridization for targeted enrichment of microbial populations. ISME J. 4, 1352–1356 (2010)
Cabello, P., Roldán, M. D. & Moreno-Vivián, C. Nitrate reduction and the nitrogen cycle in archaea. Microbiology 150, 3527–3546 (2004)
Trotsenko, Y. A. & Murrell, J. C. in Advances in Applied Microbiology Vol. 63 (eds Sariaslani, S., Laskin, A. I. & Geoffrey, M. G. ) 183–229 (Academic Press, 2008)
Nauhaus, K., Treude, T., Boetius, A. & Krüger, M. Environmental regulation of the anaerobic oxidation of methane: a comparison of ANME-I and ANME-II communities. Environ. Microbiol. 7, 98–106 (2005)
Orcutt, B., Samarkin, V., Boetius, A. & Joye, S. On the relationship between methane production and oxidation by anaerobic methanotrophic communities from cold seeps of the Gulf of Mexico. Environ. Microbiol. 10, 1108–1117 (2008)
Dekas, A. E., Poretsky, R. S. & Orphan, V. J. Deep-sea archaea fix and share nitrogen in methane-consuming microbial consortia. Science 326, 422–426 (2009)
Smith, M. R. Reversal of 2-bromoethanesulfonate inhibition of methanogenesis in Methanosarcina sp. J. Bacteriol. 156, 516–523 (1983)
Caldwell, S. L. et al. Anaerobic oxidation of methane: mechanisms, bioenergetics, and the ecology of associated microorganisms. Environ. Sci. Technol. 42, 6791–6799 (2008)
Luesken, F. A. et al. Simultaneous nitrite-dependent anaerobic methane and ammonium oxidation processes. Appl. Environ. Microbiol. 77, 6802–6807 (2011)
Brooks, P. D., Stark, J. M., McInteer, B. B. & Preston, T. Diffusion method to prepare soil extracts for automated nitrogen-15 analysis. Soil Sci. Soc. Am. J. 53, 1707–1711 (1989)
Kunin, V., Engelbrektson, A., Ochman, H. & Hugenholtz, P. Wrinkles in the rare biosphere: pyrosequencing errors can lead to artificial inflation of diversity estimates. Environ. Microbiol. 12, 118–123 (2010)
Glöckner, F. O. et al. An in situ hybridization protocol for detection and identification of planktonic bacteria. System. Applied Microbiol. 19, 403–406 (1996)
Dick, G. J. et al. Community-wide analysis of microbial genome sequence signatures. Genome Biol. 10, R85 (2009)
Markowitz, V. M. et al. IMG ER: a system for microbial genome annotation expert review and curation. Bioinformatics 25, 2271–2278 (2009)
Stewart, F. J., Ottesen, E. A. & DeLong, E. F. Development and quantitative analyses of a universal rRNA-subtraction protocol for microbial metatranscriptomics. ISME J. 4, 896–907 (2010)
Schmid, M., Schmitz-Esser, S., Jetten, M. & Wagner, M. 16S-23S rDNA intergenic spacer and 23S rDNA of anaerobic ammonium-oxidizing bacteria: implications for phylogeny and in situ detection. Environ. Microbiol. 3, 450–459 (2001)
Daims, H., Brühl, A., Amann, R., Schleifer, K.-H. & Wagner, M. The domain-specific probe EUB338 is insufficient for the detection of all bacteria: development and evaluation of a more comprehensive probe set. Systematic Applied Microbiol. 22, 434–444 (1999)
Daims, H., Lücker, S. & Wagner, M. Daime, a novel image analysis program for microbial ecology and biofilm research. Environ. Microbiol. 8, 200–213 (2006)
Caporaso, J. G. et al. QIIME allows analysis of high-throughput community sequencing data. Nature Meth. 7, 335–336 (2010)
McDonald, D. et al. An improved Greengenes taxonomy with explicit ranks for ecological and evolutionary analyses of bacteria and archaea. ISME J. 6, 610–618 (2012)
Edgar, R. C., Haas, B. J., Clemente, J. C., Quince, C. & Knight, R. UCHIME improves sensitivity and speed of chimera detection. Bioinformatics 27, 2194–2200 (2011)
Bragg, L., Stone, G., Imelfort, M., Hugenholtz, P. & Tyson, G. W. Fast, accurate error-correction of amplicon pyrosequences using Acacia. Nature Meth. 9, 425–426 (2012)
Edgar, R. C. Search and clustering orders of magnitude faster than BLAST. Bioinformatics 26, 2460–2461 (2010)
Wu, M. & Scott, A. J. Phylogenomic analysis of bacterial and archaeal sequences with AMPHORA2. Bioinformatics 28, 1033–1034 (2012)
Li, H. & Durbin, R. Fast and accurate short read alignment with Burrows–Wheeler transform. Bioinformatics 25, 1754–1760 (2009)
Li, H. et al. The Sequence Alignment/Map format and SAMtools. Bioinformatics 25, 2078–2079 (2009)
Ludwig, W. et al. ARB: a software environment for sequence data. Nucleic Acids Res. 32, 1363–1371 (2004)
DeSantis, T. Z. et al. NAST: a multiple sequence alignment server for comparative analysis of 16S rRNA genes. Nucleic Acids Res. 34, W394–W399 (2006)
Eddy, S. R. Accelerated profile HMM searches. PLOS Comput. Biol. 7, e1002195 (2011)
Price, M. N., Dehal, P. S. & Arkin, A. P. FastTree 2—approximately maximum-likelihood trees for large alignments. PLoS ONE 5, e9490 (2010)
Shi, Y., Tyson, G. W. & DeLong, E. F. Metatranscriptomics reveals unique microbial small RNAs in the ocean/'s water column. Nature 459, 266–269 (2009)
Kuenen, J. G. Anammox bacteria: from discovery to application. Nature Rev. Microbiol. 6, 320–326 (2008)
Acknowledgements
We thank the ACE sequencing team; M. Butler, F. May and S. Low for their help with the 454 pyrosequencing, Illumina and Ion Torrent sequencing and the DOE Joint Genome Institute for single-cell sequencing. We also thank P. Lu for assistance with bioreactor operation, R. Zeng and P. Lant for their contribution to the development of initial bioreactors, B. Keller, J. Li, Y. Rui and Y. Wang for chemical and isotopic analyses, and D. Willner for assistance with genomic analysis. We are grateful to J. Euzéby for etymological advice. This work is supported by the Australian Research Council (ARC) through projects DP0666762 and DP0987204 and strategic funds from the Australian Centre for Ecogenomics. G.W.T. is supported by an ARC Queen Elizabeth II Fellowship (DP1093175). P.H. is supported by an ARC Discovery Outstanding Researcher Award (DP120103498). Y.S. is supported by the China Scholarship Council.
Author information
Authors and Affiliations
Contributions
S.H. and Y.S. enriched the microorganisms in the parent bioreactor and performed batch and isotope labelling tests. S.H., Y.S., J.K. and Z.Y. performed the process data analysis. M.F.H. performed the sampling, preservation and nucleic acid extractions. M.F.H. prepared samples for single-cell genomics, metagenomic and metatranscriptomic sequencing. M.F.H. and G.W.T. performed the microbial community analysis. M.I. developed the bioinformatic tools. M.F.H., M.I., P.H. and G.W.T. performed the bioinformatics analyses. M.F.H., S.H., Z.Y., P.H., G.W.T. wrote the manuscript in consultation with all other authors.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Information
This file contains Supplementary Tables 1-3, 5, 6 and 10; full legends for Supplementary Tables 4, 7, 8 and 9 (see separate excel data files for tables); Supplementary Figures 1-14 and Supplementary References. (PDF 3183 kb)
Supplementary Data
This file contains Supplementary Table 4 (see Supplementary Information file for full legend). (XLS 379 kb)
Supplementary Data
This file contains Supplementary Table 7 (see Supplementary Information file for full legend). (XLS 545 kb)
Supplementary Data
The file contains Supplementary Table 8 (see Supplementary Information file for full legend). (XLS 27 kb)
Supplementary Data
The file contains Supplementary Table 9 (see Supplementary Information file for full legend). (XLS 27 kb)
Rights and permissions
About this article
Cite this article
Haroon, M., Hu, S., Shi, Y. et al. Anaerobic oxidation of methane coupled to nitrate reduction in a novel archaeal lineage. Nature 500, 567–570 (2013). https://doi.org/10.1038/nature12375
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nature12375
This article is cited by
-
Effects of different nitrogen applications and straw return depth on straw microbial and carbon and nitrogen cycles in paddy fields in the cool zone
Scientific Reports (2024)
-
Methane-dependent complete denitrification by a single Methylomirabilis bacterium
Nature Microbiology (2024)
-
Fertilization Methods Effect Spring Wheat Yield and Soil CH4 Fluxes in the Loess Plateau of China
International Journal of Plant Production (2024)
-
A glimpse of the paleome in endolithic microbial communities
Microbiome (2023)
-
Microbial life in 25-m-deep boreholes in ancient permafrost illuminated by metagenomics
Environmental Microbiome (2023)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.