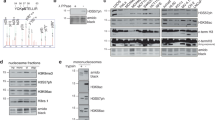
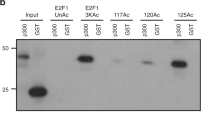
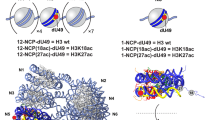
Abstract
The detection of DNA lesions within chromatin represents a critical step in cellular responses to DNA damage. However, the regulatory mechanisms that couple chromatin sensing to DNA-damage signalling in mammalian cells are not well understood. Here we show that tyrosine phosphorylation of the protein acetyltransferase KAT5 (also known as TIP60) increases after DNA damage in a manner that promotes KAT5 binding to the histone mark H3K9me3. This triggers KAT5-mediated acetylation of the ATM kinase, promoting DNA-damage-checkpoint activation and cell survival. We also establish that chromatin alterations can themselves enhance KAT5 tyrosine phosphorylation and ATM-dependent signalling, and identify the proto-oncogene c-Abl as a mediator of this modification. These findings define KAT5 tyrosine phosphorylation as a key event in the sensing of genomic and chromatin perturbations, and highlight a key role for c-Abl in such processes.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Jackson, S. P. & Bartek, J. The DNA-damage response in human biology and disease. Nature 461, 1071–1078 (2009)
Harper, J. W. & Elledge, S. J. The DNA damage response: ten years after. Mol. Cell 28, 739–745 (2007)
Miller, K. M. & Jackson, S. P. Histone marks: repairing DNA breaks within the context of chromatin. Biochem. Soc. Trans. 40, 370–376 (2012)
Downs, J. A., Nussenzweig, M. C. & Nussenzweig, A. Chromatin dynamics and the preservation of genetic information. Nature 447, 951–958 (2007)
Bartek, J. & Lukas, J. DNA damage checkpoints: from initiation to recovery or adaptation. Curr. Opin. Cell Biol. 19, 238–245 (2007)
Shiloh, Y. The ATM-mediated DNA-damage response: taking shape. Trends Biochem. Sci. 31, 402–410 (2006)
Lee, J.-H. & Paull, T. T. Direct activation of the ATM protein kinase by the Mre11/Rad50/Nbs1 complex. Science 304, 93–96 (2004)
Lee, J.-H. & Paull, T. T. ATM activation by DNA double-strand breaks through the Mre11-Rad50-Nbs1 complex. Science 308, 551–554 (2005)
Falck, J., Coates, J. & Jackson, S. P. Conserved modes of recruitment of ATM, ATR and DNA-PKcs to sites of DNA damage. Nature 434, 605–611 (2005)
Uziel, T. et al. Requirement of the MRN complex for ATM activation by DNA damage. EMBO J. 22, 5612–5621 (2003)
Ayoub, N., Jeyasekharan, A. D., Bernal, J. A. & Venkitaraman, A. R. HP1-beta mobilization promotes chromatin changes that initiate the DNA damage response. Nature 453, 682–686 (2008)
Sun, Y. et al. Histone H3 methylation links DNA damage detection to activation of the tumour suppressor Tip60. Nature Cell Biol. 11, 1376–1382 (2009)
Sun, Y., Xu, Y., Roy, K. & Price, B. D. DNA damage-induced acetylation of lysine 3016 of ATM activates ATM kinase activity. Mol. Cell. Biol. 27, 8502–8509 (2007)
Kimura, A. & Horikoshi, M. Tip60 acetylates six lysines of a specific class in core histones in vitro. Genes Cells 3, 789–800 (1998)
Sun, Y., Jiang, X., Chen, S., Fernandes, N. & Price, B. D. A role for the Tip60 histone acetyltransferase in the acetylation and activation of ATM. Proc. Natl Acad. Sci. USA 102, 13182–13187 (2005)
Ziv, Y. et al. Chromatin relaxation in response to DNA double-strand breaks is modulated by a novel ATM- and KAP-1 dependent pathway. Nature Cell Biol. 8, 870–876 (2006)
Kruhlak, M. J. et al. Changes in chromatin structure and mobility in living cells at sites of DNA double-strand breaks. J. Cell Biol. 172, 823–834 (2006)
Bakkenist, C. J. & Kastan, M. B. DNA damage activates ATM through intermolecular autophosphorylation and dimer dissociation. Nature 421, 499–506 (2003)
Hickson, I. et al. Identification and characterization of a novel and specific inhibitor of the ataxia-telangiectasia mutated kinase ATM. Cancer Res. 64, 9152–9159 (2004)
Shafman, T. et al. Interaction between ATM protein and c-Abl in response to DNA damage. Nature 387, 520–523 (1997)
Meltser, V., Ben-Yehoyada, M. & Shaul, Y. c-Abl tyrosine kinase in the DNA damage response: cell death and more. Cell Death Differ. 18, 2–4 (2011)
Wang, X. et al. A positive role for c-Abl in Atm and Atr activation in DNA damage response. Cell Death Differ. 18, 5–15 (2011)
Kharbanda, S. et al. Activation of the c-Abl tyrosine kinase in the stress response to DNA-damaging agents. Nature 376, 785–788 (1995)
Baskaran, R. et al. Ataxia telangiectasia mutant protein activates c-Abl tyrosine kinase in response to ionizing radiation. Nature 387, 516–519 (1997)
Buchdunger, E. et al. Inhibition of the Abl protein-tyrosine kinase in vitro and in vivo by a 2-phenylaminopyrimidine derivative. Cancer Res. 56, 100–104 (1996)
Mikkelsen, T. S. et al. Genome-wide maps of chromatin state in pluripotent and lineage-committed cells. Nature 448, 553–560 (2007)
Edmunds, J. W., Mahadevan, L. C. & Clayton, A. L. Dynamic histone H3 methylation during gene induction: HYPB/Setd2 mediates all H3K36 trimethylation. EMBO J. 27, 406–420 (2008)
Chantalat, S. et al. Histone H3 trimethylation at lysine 36 is associated with constitutive and facultative heterochromatin. Genome Res. 21, 1426–1437 (2011)
Jiang, Z. et al. Tip60-mediated acetylation activates transcription independent apoptotic activity of Abl. Mol. Cancer 10, 88 (2011)
Brasher, B. B. & Van Etten, R. A. c-Abl has high intrinsic tyrosine kinase activity that is stimulated by mutation of the Src homology 3 domain and by autophosphorylation at two distinct regulatory tyrosines. J. Biol. Chem. 275, 35631–35637 (2000)
Scaffidi, P. & Misteli, T. Lamin A-dependent nuclear defects in human aging. Science 312, 1059–1063 (2006)
Pegoraro, G. & Misteli, T. The central role of chromatin maintenance in aging. Aging (Albany NY) 1, 1017–1022 (2009)
Pegoraro, G. et al. Ageing-related chromatin defects through loss of the NURD complex. Nature Cell Biol. 11, 1261–1267 (2009)
Lane, A. A. & Chabner, B. A. Histone deacetylase inhibitors in cancer therapy. J. Clin. Oncol. 27, 5459–5468 (2009)
Podtcheko, A. et al. Inhibition of ABL tyrosine kinase potentiates radiation-induced terminal growth arrest in anaplastic thyroid cancer cells. Radiat. Res. 165, 35–42 (2006)
Acknowledgements
We thank all members of the Jackson laboratory for help and support, and A. Blackford, S. Britton, K. Dry, J. Forment and J. Travers for critical reading of the manuscript. Research in the Jackson laboratory is funded by Cancer Research UK program grant C6/A11224, the European Research Council and the European Community Seventh Framework Programme grant agreement no. HEALTH-F2-2010-259893 (DDR). Core funding is provided by CRUK (C6946/A14492) and the Wellcome Trust (WT092096). S.P.J. receives his salary from the University of Cambridge, UK, supplemented by CRUK. A.K. is funded by a Herchel Smith Fellowship from the University of Cambridge.
Author information
Authors and Affiliations
Contributions
All experiments were conceived by A.K. and S.P.J. and were carried out by A.K. S.P.J. and A.K. wrote the paper.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Information
This file contains Supplementary Figures 1-15 and Supplementary Tables 1-2. (PDF 1556 kb)
Rights and permissions
About this article
Cite this article
Kaidi, A., Jackson, S. KAT5 tyrosine phosphorylation couples chromatin sensing to ATM signalling. Nature 498, 70–74 (2013). https://doi.org/10.1038/nature12201
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nature12201
This article is cited by
-
LOXL2-mediated H3K4 oxidation reduces chromatin accessibility in triple-negative breast cancer cells
Oncogene (2020)
-
Regulation of DNA damage-induced ATM activation by histone modifications
Genome Instability & Disease (2020)
-
Quantifying Tip60 (Kat5) stratifies breast cancer
Scientific Reports (2019)
-
The CHD6 chromatin remodeler is an oxidative DNA damage response factor
Nature Communications (2019)
-
Radiation-dose-dependent functional synergisms between ATM, ATR and DNA-PKcs in checkpoint control and resection in G2-phase
Scientific Reports (2019)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.