Abstract
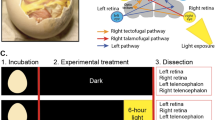
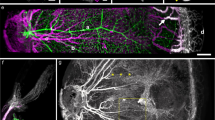
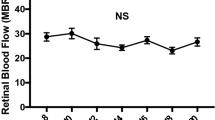
Vascular patterning is critical for organ function. In the eye, there is simultaneous regression of embryonic hyaloid vasculature1 (important to clear the optical path) and formation of the retinal vasculature2 (important for the high metabolic demands of retinal neurons). These events occur postnatally in the mouse. Here we have identified a light-response pathway that regulates both processes. We show that when mice are mutated in the gene (Opn4) for the atypical opsin melanopsin3,4,5, or are dark-reared from late gestation, the hyaloid vessels are persistent at 8 days post-partum and the retinal vasculature overgrows. We provide evidence that these vascular anomalies are explained by a light-response pathway that suppresses retinal neuron number, limits hypoxia and, as a consequence, holds local expression of vascular endothelial growth factor (VEGFA) in check. We also show that the light response for this pathway occurs in late gestation at about embryonic day 16 and requires the photopigment in the fetus and not the mother. Measurements show that visceral cavity photon flux is probably sufficient to activate melanopsin-expressing retinal ganglion cells in the mouse fetus. These data thus show that light—the stimulus for function of the mature eye—is also critical in preparing the eye for vision by regulating retinal neuron number and initiating a series of events that ultimately pattern the ocular blood vessels.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Ito, M. & Yoshioka, M. Regression of the hyaloid vessels and pupillary membrane of the mouse. Anat. Embryol. (Berl.) 200, 403–411 (1999)
Fruttiger, M. Development of the retinal vasculature. Angiogenesis 10, 77–88 (2007)
Provencio, I., Jiang, G., De Grip, W. J., Hayes, W. P. & Rollag, M. D. Melanopsin: an opsin in melanophores, brain, and eye. Proc. Natl Acad. Sci. USA 95, 340–345 (1998)
Hattar, S., Liao, H. W., Takao, M., Berson, D. M. & Yau, K. W. Melanopsin-containing retinal ganglion cells: architecture, projections, and intrinsic photosensitivity. Science 295, 1065–1070 (2002)
Panda, S. et al. Melanopsin (Opn4) requirement for normal light-induced circadian phase shifting. Science 298, 2213–2216 (2002)
Johnson, J. et al. Melanopsin-dependent light avoidance in neonatal mice. Proc. Natl Acad. Sci. USA 107, 17374–17378 (2010)
Tian, N. & Copenhagen, D. R. Visual stimulation is required for refinement of ON and OFF pathways in postnatal retina. Neuron 39, 85–96 (2003)
Diez-Roux, G. & Lang, R. A. Macrophages induce apoptosis in normal cells in vivo. Development 124, 3633–3638 (1997)
Lobov, I. B. et al. WNT7b mediates macrophage-induced programmed cell death in patterning of the vasculature. Nature 437, 417–421 (2005)
Saint-Geniez, M. & D’Amore, P. A. Development and pathology of the hyaloid, choroidal and retinal vasculature. Int. J. Dev. Biol. 48, 1045–1058 (2004)
Tarttelin, E. E. et al. Expression of opsin genes early in ocular development of humans and mice. Exp. Eye Res. 76, 393–396 (2003)
Do, M. T. & Yau, K. W. Intrinsically photosensitive retinal ganglion cells. Physiol. Rev. 90, 1547–1581 (2010)
Edwards, M. M. et al. The deletion of Math5 disrupts retinal blood vessel and glial development in mice. Exp. Eye Res. 96, 147–156 (2012)
Ferrara, N., Gerber, H. P. & LeCouter, J. The biology of VEGF and its receptors. Nature Med. 9, 669–676 (2003)
Haigh, J. J. et al. Cortical and retinal defects caused by dosage-dependent reductions in VEGF-A paracrine signaling. Dev. Biol. 262, 225–241 (2003)
Meeson, A. P., Argilla, M., Ko, K., Witte, L. & Lang, R. A. VEGF deprivation-induced apoptosis is a component of programmed capillary regression. Development 126, 1407–1415 (1999)
Adamis, A. P. et al. Increased vascular endothelial growth factor levels in the vitreous of eyes with proliferative diabetic retinopathy. Am. J. Ophthalmol. 118, 445–450 (1994)
Rowan, S. & Cepko, C. L. Genetic analysis of the homeodomain transcription factor Chx10 in the retina using a novel multifunctional BAC transgenic mouse reporter. Dev. Biol. 271, 388–402 (2004)
Kurihara, T. et al. von Hippel-Lindau protein regulates transition from the fetal to the adult circulatory system in retina. Development 137, 1563–1571 (2010)
Lange, C. et al. Retina-specific activation of a sustained hypoxia-like response leads to severe retinal degeneration and loss of vision. Neurobiol. Dis. 41, 119–130 (2010)
Sekaran, S. et al. Melanopsin-dependent photoreception provides earliest light detection in the mammalian retina. Curr. Biol. 15, 1099–1107 (2005)
Tu, D. C. et al. Physiologic diversity and development of intrinsically photosensitive retinal ganglion cells. Neuron 48, 987–999 (2005)
Wong, K. Y. A retinal ganglion cell that can signal irradiance continuously for 10 hours. J. Neurosci. 32, 11478–11485 (2012)
Connor, K. M. et al. Quantification of oxygen-induced retinopathy in the mouse: a model of vessel loss, vessel regrowth and pathological angiogenesis. Nature Protocols 4, 1565–1573 (2009)
Stefater, J. A., III et al. Regulation of angiogenesis by a non-canonical Wnt–Flt1 pathway in myeloid cells. Nature 474, 511–515 (2011)
Lichtenberger, B. M. et al. Autocrine VEGF signaling synergizes with EGFR in tumor cells to promote epithelial cancer development. Cell 140, 268–279 (2010)
Ecker, J. L. et al. Melanopsin-expressing retinal ganglion-cell photoreceptors: cellular diversity and role in pattern vision. Neuron 67, 49–60 (2010)
Madisen, L. et al. A robust and high-throughput Cre reporting and characterization system for the whole mouse brain. Nature Neurosci. 13, 133–140 (2010)
Gerhardt, H. et al. VEGF guides angiogenic sprouting utilizing endothelial tip cell filopodia. J. Cell Biol. 161, 1163–1177 (2003)
Stockmann, C. et al. Deletion of vascular endothelial growth factor in myeloid cells accelerates tumorigenesis. Nature 456, 814–818 (2008)
Goldberg, J. L., Klassen, M. P., Hua, Y. & Barres, B. A. Amacrine-signaled loss of intrinsic axon growth ability by retinal ganglion cells. Science 296, 1860–1864 (2002)
Acknowledgements
We thank D. Bredl, P. Speeg and M. Sarangdhar for technical assistance, A. Delwig and N. Brown for advice. We acknowledge the assistance of the Research Flow Cytometry Core in the Division of Rheumatology at CCHMC, supported in part by NIH AR-47363. This work was supported by the NIH (R.A.L., D.R.C., J.M.K. and R.S.H.) with additional funding from the Abrahamson Pediatric Eye Institute of CCHMC, That Man May See at UCSF, Research to Prevent Blindness (D.R.C.) and March of Dimes (D.R.C.).
Author information
Authors and Affiliations
Contributions
R.A.L. and D.R.C. provided project leadership. R.A.L., D.R.C. and S.R. wrote the manuscript. R.A.L., D.R.C. and R.S.H. supervised experimental work. N.F. developed critical reagents and M.B.Y. provided important clinical insights. S.R., C.C., J.F., J.M.K. and D.R.C. performed experimentation and analysis.
Corresponding authors
Ethics declarations
Competing interests
N.F. is an employee of Genentech Corporation.
Supplementary information
Supplementary Information
This file contains Supplementary Text, Supplementary Figures 1-7 and Supplementary References. (PDF 663 kb)
Rights and permissions
About this article
Cite this article
Rao, S., Chun, C., Fan, J. et al. A direct and melanopsin-dependent fetal light response regulates mouse eye development. Nature 494, 243–246 (2013). https://doi.org/10.1038/nature11823
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nature11823
This article is cited by
-
Dopamine modulates the retinal clock through melanopsin-dependent regulation of cholinergic waves during development
BMC Biology (2023)
-
Biologically aware lighting for newborn intensive care
Journal of Perinatology (2023)
-
Neuronal Bmal1 regulates retinal angiogenesis and neovascularization in mice
Communications Biology (2022)
-
Illuminating brain development
Cell Research (2022)
-
Diversity of intrinsically photosensitive retinal ganglion cells: circuits and functions
Cellular and Molecular Life Sciences (2021)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.