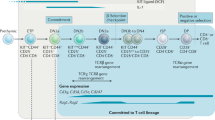
Abstract
Although most genes are expressed biallelically, a number of key genomic sites—including immune and olfactory receptor regions—are controlled monoallelically in a stochastic manner, with some cells expressing the maternal allele and others the paternal allele in the target tissue1,2. Very little is known about how this phenomenon is regulated and programmed during development. Here, using mouse immunoglobulin-κ (Igκ) as a model system, we demonstrate that although individual haematopoietic stem cells are characterized by allelic plasticity, early lymphoid lineage cells become committed to the choice of a single allele, and this decision is then stably maintained in a clonal manner that predetermines monoallelic rearrangement in B cells. This is accompanied at the molecular level by underlying allelic changes in asynchronous replication timing patterns at the κ locus. These experiments may serve to define a new concept of stem cell plasticity.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Chess, A., Simon, I., Cedar, H. & Axel, R. Allelic inactivation regulates olfactory receptor gene expression. Cell 78, 823–834 (1994)
Mostoslavsky, R. et al. Asynchronous replication and allelic exclusion in the immune system. Nature 414, 221–225 (2001)
Casellas, R. et al. Contribution of receptor editing to the antibody repertoire. Science 291, 1541–1544 (2001)
Goldmit, M. et al. Epigenetic ontogeny of the Igκ locus during B cell development. Nature Immunol. 6, 198–203 (2005)
Fitzsimmons, S. P., Bernstein, R. M., Max, E. E., Skok, J. A. & Shapiro, M. A. Dynamic changes in accessibility, nuclear positioning, recombination, and transcription at the Igκ locus. J. Immunol. 179, 5264–5273 (2007)
Rolink, A., Kudo, A., Karasuyama, H., Kikuchi, Y. & Melchers, F. Long-term proliferating early pre-B cell lines and clones with the potential to develop to surface Ig-positive, mitogen reactive B cells in vitro and in vivo. EMBO J. 10, 327–336 (1991)
Ji, Y. et al. The in vivo pattern of binding of RAG1 and RAG2 to antigen receptor loci. Cell 141, 419–431 (2010)
Matthews, A. G. et al. RAG2 PHD finger couples histone H3 lysine 4 trimethylation with V(D)J recombination. Nature 450, 1106–1110 (2007)
Liu, Y., Subrahmanyam, R., Chakraborty, T., Sen, R. & Desiderio, S. A plant homeodomain in RAG-2 that binds hypermethylated lysine 4 of histone H3 is necessary for efficient antigen-receptor-gene rearrangement. Immunity 27, 561–571 (2007)
Medvedovic, J., Ebert, A., Tagoh, H. & Busslinger, M. Pax5: a master regulator of B cell development and leukemogenesis. Adv. Immunol. 111, 179–206 (2011)
Ramírez, J., Lukin, K. & Hagman, J. From hematopoietic progenitors to B cells: mechanisms of lineage restriction and commitment. Curr. Opin. Immunol. 22, 177–184 (2010)
Goren, A. & Cedar, H. Replicating by the clock. Nature Rev. Mol. Cell Biol. 4, 25–32 (2003)
Singh, N. et al. Coordination of the random asynchronous replication of autosomal loci. Nature Genet. 33, 339–341 (2003)
Rountree, M. R., Bachman, K. E. & Baylin, S. B. DNMT1 binds HDAC2 and a new co-repressor, DMAP1, to form a complex at replication foci. Nature Genet. 25, 269–277 (2000)
Zhang, J., Feng, X., Hashimshony, T., Keshet, I. & Cedar, H. Establishment of transcriptional competence in early and late S-phase. Nature 420, 198–202 (2002)
Lande-Diner, L., Zhang, J. & Cedar, H. Shifts in replication timing actively affect histone acetylation during nucleosome reassembly. Mol. Cell 34, 767–774 (2009)
Goren, A., Tabib, A., Hecht, M. & Cedar, H. DNA replication timing of the human β-globin domain is controlled by histone modification at the origin. Genes Dev. 22, 1319–1324 (2008)
Lande-Diner, L. & Cedar, H. Silence of the genes—mechanisms of long term repression. Nature Rev. Genet. 6, 648–654 (2005)
Frommer, F. et al. Tolerance without clonal expansion: self-antigen-expressing B cells program self-reactive T cells for future deletion. J. Immunol. 181, 5748–5759 (2008)
Gribnau, J., Luikenhuis, S., Hochedlinger, K., Monkhorst, K. & Jaenisch, R. X chromosome choice occurs independently of asynchronous replication timing. J. Cell Biol. 168, 365–373 (2005)
Alexander, M. K. et al. Differences between homologous alleles of olfactory receptor genes require the polycomb group protein Eed. J. Cell Biol. 179, 269–276 (2007)
Mostoslavsky, G. et al. Efficiency of transduction of highly purified murine hematopoietic stem cells by lentiviral and oncoretroviral vectors under conditions of minimal in vitro manipulation. Mol. Ther. 11, 932–940 (2005)
Ikawa, M., Yamada, S., Nakanishi, T. & Okabe, M. Green fluorescent protein (GFP) as a vital marker in mammals. Curr. Top. Dev. Biol. 44, 1–20 (1999)
Serwold, T., Hochedlinger, K., Inlay, M. A., Jaenisch, R. & Weissman, I. L. Early TCR expression and aberrant T cell development in mice with endogenous prerearranged T cell receptor genes. J. Immunol. 179, 928–938 (2007)
Simon, I. et al. Asynchronous replication of imprinted genes is established in the gametes and maintained during development. Nature 401, 929–932 (1999)
Gimelbrant, A. A., Ensminger, A. W., Qi, P., Zucker, J. & Chess, A. Monoallelic expression and asynchronous replication of p120 catenin in mouse and human cells. J. Biol. Chem. 280, 1354–1359 (2005)
Lemischka, I. R., Raulet, D. H. & Mulligan, R. C. Developmental potential and dynamic behavior of hematopoietic stem cells. Cell 45, 917–927 (1986)
Cedar, H. & Bergman, Y. Epigenetics of hematopoietic cell development. Nature Rev. Immunol. 11, 478–488 (2011)
Epsztejn-Litman, S. et al. De novo DNA methylation promoted by G9a prevents reprogramming of embryonically silenced genes. Nature Struct. Mol. Biol. 15, 1176–1183 (2008)
Schmidt, M. et al. High-resolution insertion-site analysis by linear amplification-mediated PCR (LAM-PCR). Nature Methods 4, 1051–1057 (2007)
Hochedlinger, K. & Jaenisch, R. Monoclonal mice generated by nuclear transfer from mature B and T donor cells. Nature 415, 1035–1038 (2002)
Schlesinger, S., Selig, S., Bergman, Y. & Cedar, H. Allelic inactivation of rDNA loci. Genes Dev. 23, 2437–2447 (2009)
Hanna, J. et al. Direct reprogramming of terminally differentiated mature B lymphocytes to pluripotency. Cell 133, 250–264 (2008)
Liu, X. & Van Ness, B. Gene targeting of the KI–KII sequence elements in a model pre-B cell line: effects on germline transcription and rearrangement of the κ locus. Mol. Immunol. 36, 461–469 (1999)
Novobrantseva, T. I. et al. Rearrangement and expression of immunoglobulin light chain genes can precede heavy chain expression during normal B cell development in mice. J. Exp. Med. 189, 75–88 (1999)
Mostoslavsky, G., Fabian, A. J., Rooney, S., Alt, F. W. & Mulligan, R. C. Complete correction of murine Artemis immunodeficiency by lentiviral vector-mediated gene transfer. Proc. Natl Acad. Sci. USA 103, 16406–16411 (2006)
Acknowledgements
We thank M. Nussenzweig for providing the Igκm/h mice, A. Waisman for providing the IiMOG-marked ES cells, A. G. Fisher and V. Azuara for guiding us through the replication timing analysis by S-phase fractionation assay, M. Inlay for coaching us on the purification and culturing of MPPs and CLPs, A. Wutz for providing some of the single-cell clones, Y. Smith for image analysis and L. Dempsey for sharing ideas. This work was supported by research grants from the Israel Academy of Sciences (H.C., Y.B.), National Institutes of Health (Y.B.), the Israel Cancer Research Foundation (H.C., Y.B.), the European Community 5th Framework Quality of Life Program (Y.B.), Lew Sanders (H.C.) and Norton Herrick (H.C.).
Author information
Authors and Affiliations
Contributions
M.F. and C.R. designed the experiments, did the research, interpreted the results and assisted in writing the manuscript; M.T. initiated the allelic ChIP and DNA replication analyses; S.F. performed the allelic VJκ rearrangement in B6/Cast clones; S.S. studied asynchronous replication in ES cells; H.M. studied asynchronous replication in HSCs, MPPs and CLPs; G.T. and D.S. helped with Rag2 ChIP assays; Y.R. and J.H.H. generated the LN3 (Vβ1NT/+) mice from the appropriate ES cells; M.G., A.M. and S.J. designed and helped with the MPP and CLP reconstitution, and FACS experiments; G.M. designed and helped with the HSCs, pro-B, MPP and CLP reconstitution experiments; and H.C. and Y.B. directed the study and wrote the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Information
This file contains Supplementary Figures 1-9, Supplementary Tables 1-4 and additional references. (PDF 2385 kb)
Rights and permissions
About this article
Cite this article
Farago, M., Rosenbluh, C., Tevlin, M. et al. Clonal allelic predetermination of immunoglobulin-κ rearrangement. Nature 490, 561–565 (2012). https://doi.org/10.1038/nature11496
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nature11496
This article is cited by
-
Chromosomal coordination and differential structure of asynchronous replicating regions
Nature Communications (2021)
-
Clonally stable Vκ allelic choice instructs Igκ repertoire
Nature Communications (2017)
-
Programming asynchronous replication in stem cells
Nature Structural & Molecular Biology (2017)
-
Landscape of monoallelic DNA accessibility in mouse embryonic stem cells and neural progenitor cells
Nature Genetics (2017)
-
Chromosome choice for initiation of V–(D)–J recombination is not governed by genomic imprinting
Immunology & Cell Biology (2017)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.