Abstract
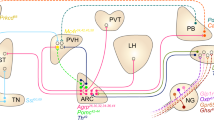
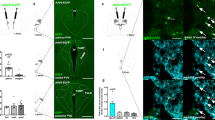
Hunger is a complex behavioural state that elicits intense food seeking and consumption. These behaviours are rapidly recapitulated by activation of starvation-sensitive AGRP neurons, which present an entry point for reverse-engineering neural circuits for hunger. Here we mapped synaptic interactions of AGRP neurons with multiple cell populations in mice and probed the contribution of these distinct circuits to feeding behaviour using optogenetic and pharmacogenetic techniques. An inhibitory circuit with paraventricular hypothalamus (PVH) neurons substantially accounted for acute AGRP neuron-evoked eating, whereas two other prominent circuits were insufficient. Within the PVH, we found that AGRP neurons target and inhibit oxytocin neurons, a small population that is selectively lost in Prader–Willi syndrome, a condition involving insatiable hunger. By developing strategies for evaluating molecularly defined circuits, we show that AGRP neuron suppression of oxytocin neurons is critical for evoked feeding. These experiments reveal a new neural circuit that regulates hunger state and pathways associated with overeating disorders.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Cowley, M. A. et al. The distribution and mechanism of action of ghrelin in the CNS demonstrates a novel hypothalamic circuit regulating energy homeostasis. Neuron 37, 649–661 (2003)
Andrews, Z. B. et al. UCP2 mediates ghrelin’s action on NPY/AgRP neurons by lowering free radicals. Nature 454, 846–851 (2008)
Yang, Y., Atasoy, D., Su, H. H. & Sternson, S. M. Hunger states switch a flip-flop memory circuit via a synaptic AMPK-dependent positive feedback loop. Cell 146, 992–1003 (2011)
Aponte, Y., Atasoy, D. & Sternson, S. M. AGRP neurons are sufficient to orchestrate feeding behavior rapidly and without training. Nature Neurosci. 14, 351–355 (2011)
Krashes, M. J. et al. Rapid, reversible activation of AgRP neurons drives feeding behavior in mice. J. Clin. Invest. 121, 1424–1428 (2011)
Luquet, S., Perez, F. A., Hnasko, T. S. & Palmiter, R. D. NPY/AgRP neurons are essential for feeding in adult mice but can be ablated in neonates. Science 310, 683–685 (2005)
Cannon, W. B. The Wisdom of the Body 2nd edn (W.W. Norton & Co., 1939)
Broberger, C., Johansen, J., Johansson, C., Schalling, M. & Hokfelt, T. The neuropeptide Y/agouti gene-related protein (AGRP) brain circuitry in normal, anorectic, and monosodium glutamate-treated mice. Proc. Natl Acad. Sci. USA 95, 15043–15048 (1998)
Gropp, E. et al. Agouti-related peptide-expressing neurons are mandatory for feeding. Nature Neurosci. 8, 1289–1291 (2005)
Yaswen, L., Diehl, N., Brennan, M. B. & Hochgeschwender, U. Obesity in the mouse model of pro-opiomelanocortin deficiency responds to peripheral melanocortin. Nature Med. 5, 1066–1070 (1999)
Wu, Q., Boyle, M. P. & Palmiter, R. D. Loss of GABAergic signaling by AgRP neurons to the parabrachial nucleus leads to starvation. Cell 137, 1225–1234 (2009)
Wu, Q., Clark, M. S. & Palmiter, R. D. Deciphering a neuronal circuit that mediates appetite. Nature 483, 594–597 (2012)
Leibowitz, S. F., Hammer, N. J. & Chang, K. Hypothalamic paraventricular nucleus lesions produce overeating and obesity in the rat. Physiol. Behav. 27, 1031–1040 (1981)
Balthasar, N. et al. Divergence of melanocortin pathways in the control of food intake and energy expenditure. Cell 123, 493–505 (2005)
Xi, D., Gandhi, N., Lai, M. & Kublaoui, B. M. Ablation of Sim1 neurons causes obesity through hyperphagia and reduced energy expenditure. PLoS ONE 7, e36453 (2012)
Avery, L. & Wasserman, S. Ordering gene function: the interpretation of epistasis in regulatory hierarchies. Trends Genet. 8, 312–316 (1992)
Collins, S. R. et al. Functional dissection of protein complexes involved in yeast chromosome biology using a genetic interaction map. Nature 446, 806–810 (2007)
Cowley, M. A. et al. Leptin activates anorexigenic POMC neurons through a neural network in the arcuate nucleus. Nature 411, 480–484 (2001)
Tong, Q., Ye, C. P., Jones, J. E., Elmquist, J. K. & Lowell, B. B. Synaptic release of GABA by AgRP neurons is required for normal regulation of energy balance. Nature Neurosci. 11, 998–1000 (2008)
Petreanu, L., Huber, D., Sobczyk, A. & Svoboda, K. Channelrhodopsin-2-assisted circuit mapping of long-range callosal projections. Nature Neurosci. 10, 663–668 (2007)
Boyden, E. S., Zhang, F., Bamberg, E., Nagel, G. & Deisseroth, K. Millisecond-timescale, genetically targeted optical control of neural activity. Nature Neurosci. 8, 1263–1268 (2005)
Atasoy, D., Aponte, Y., Su, H. H. & Sternson, S. M. A. FLEX switch targets channelrhodopsin-2 to multiple cell types for imaging and long-range circuit mapping. J. Neurosci. 28, 7025–7030 (2008)
Dicken, M. S., Tooker, R. E. & Hentges, S. T. Regulation of GABA and glutamate release from proopiomelanocortin neuron terminals in intact hypothalamic networks. J. Neurosci. 32, 4042–4048 (2012)
Armbruster, B. N., Li, X., Pausch, M. H., Herlitze, S. & Roth, B. L. Evolving the lock to fit the key to create a family of G protein-coupled receptors potently activated by an inert ligand. Proc. Natl Acad. Sci. USA 104, 5163–5168 (2007)
Higgs, S. & Cooper, S. J. Hyperphagia induced by direct administration of midazolam into the parabrachial nucleus of the rat. Eur. J. Pharmacol. 313, 1–9 (1996)
Hefft, S. & Jonas, P. Asynchronous GABA release generates long-lasting inhibition at a hippocampal interneuron-principal neuron synapse. Nature Neurosci. 8, 1319–1328 (2005)
Best, A. R. & Regehr, W. G. Inhibitory regulation of electrically coupled neurons in the inferior olive is mediated by asynchronous release of GABA. Neuron 62, 555–565 (2009)
Kelly, J., Rothstein, J. & Grossman, S. P. GABA and hypothalamic feeding systems. I. Topographic analysis of the effects of microinjections of muscimol. Physiol. Behav. 23, 1123–1134 (1979)
Kublaoui, B. M., Gemelli, T., Tolson, K. P., Wang, Y. & Zinn, A. R. Oxytocin deficiency mediates hyperphagic obesity of Sim1 haploinsufficient mice. Mol. Endocrinol. 22, 1723–1734 (2008)
Magnus, C. J. et al. Chemical and genetic engineering of selective ion channel-ligand interactions. Science 333, 1292–1296 (2011)
Hodos, W. Progressive ratio as a measure of reward strength. Science 134, 943–944 (1961)
Swanson, L. W. & Sawchenko, P. E. Hypothalamic integration: organization of the paraventricular and supraoptic nuclei. Annu. Rev. Neurosci. 6, 269–324 (1983)
Biag, J. et al. Cyto- and chemoarchitecture of the hypothalamic paraventricular nucleus in the C57BL/6J male mouse: a study of immunostaining and multiple fluorescent tract tracing. J. Comp. Neurol. 520, 6–33 (2012)
Swaab, D. F., Purba, J. S. & Hofman, M. A. Alterations in the hypothalamic paraventricular nucleus and its oxytocin neurons (putative satiety cells) in Prader–Willi syndrome: a study of five cases. J. Clin. Endocrinol. Metab. 80, 573–579 (1995)
Holder, J. L., Jr, Butte, N. F. & Zinn, A. R. Profound obesity associated with a balanced translocation that disrupts the SIM1 gene. Hum. Mol. Genet. 9, 101–108 (2000)
Traurig, M. et al. Common variation in SIM1 is reproducibly associated with BMI in Pima Indians. Diabetes 58, 1682–1689 (2009)
Fields, R. L., Ponzio, T. A., Kawasaki, M. & Gainer, H. Cell-type specific oxytocin gene expression from AAV delivered promoter deletion constructs into the rat supraoptic nucleus in vivo . PLoS ONE 7, e32085 (2012)
Knobloch, H. S. et al. Evoked axonal oxytocin release in the central amygdala attenuates fear response. Neuron 73, 553–566 (2012)
Clark, J. T., Kalra, P. S., Crowley, W. R. & Kalra, S. P. Neuropeptide Y and human pancreatic polypeptide stimulate feeding behavior in rats. Endocrinology 115, 427–429 (1984)
Arletti, R., Benelli, A. & Bertolini, A. Oxytocin inhibits food and fluid intake in rats. Physiol. Behav. 48, 825–830 (1990)
Swanson, L. W., Sawchenko, P. E., Wiegand, S. J. & Price, J. L. Separate neurons in the paraventricular nucleus project to the median eminence and to the medulla or spinal cord. Brain Res. 198, 190–195 (1980)
Blevins, J. E., Eakin, T. J., Murphy, J. A., Schwartz, M. W. & Baskin, D. G. Oxytocin innervation of caudal brainstem nuclei activated by cholecystokinin. Brain Res. 993, 30–41 (2003)
Zhang, G. et al. Neuropeptide exocytosis involving synaptotagmin-4 and oxytocin in hypothalamic programming of body weight and energy balance. Neuron 69, 523–535 (2011)
Baskin, D. G. et al. A new oxytocin-saporin cytotoxin for lesioning oxytocin-receptive neurons in the rat hindbrain. Endocrinology 151, 4207–4213 (2010)
Kaelin, C. B., Xu, A. W., Lu, X. Y. & Barsh, G. S. Transcriptional regulation of agouti-related protein (Agrp) in transgenic mice. Endocrinology 145, 5798–5806 (2004)
Balthasar, N. et al. Leptin receptor signaling in POMC neurons is required for normal body weight homeostasis. Neuron 42, 983–991 (2004)
Young, W. S., III et al. Transgenic expression of green fluorescent protein in mouse oxytocin neurones. J. Neuroendocrinol. 11, 935–939 (1999)
Pinto, S. et al. Rapid rewiring of arcuate nucleus feeding circuits by leptin. Science 304, 110–115 (2004)
Erickson, J. C., Clegg, K. E. & Palmiter, R. D. Sensitivity to leptin and susceptibility to seizures of mice lacking neuropeptide Y. Nature 381, 415–418 (1996)
Aravanis, A. M. et al. An optical neural interface: in vivo control of rodent motor cortex with integrated fiberoptic and optogenetic technology. J. Neural Eng. 4, S143–S156 (2007)
Wieland, H. A., Engel, W., Eberlein, W., Rudolf, K. & Doods, H. N. Subtype selectivity of the novel nonpeptide neuropeptide Y Y1 receptor antagonist BIBO 3304 and its effect on feeding in rodents. Br. J. Pharmacol. 125, 549–555 (1998)
Zhang, M., Balmadrid, C. & Kelley, A. E. Nucleus accumbens opioid, GABaergic, and dopaminergic modulation of palatable food motivation: contrasting effects revealed by a progressive ratio study in the rat. Behav. Neurosci. 117, 202–211 (2003)
Peng, H., Ruan, Z., Atasoy, D. & Sternson, S. Automatic reconstruction of 3D neuron structures using a graph-augmented deformable model. Bioinformatics 26, i38–i46 (2010).
Acknowledgements
This research was funded by the Howard Hughes Medical Institute. We thank J. Cox, A. Wardlaw, K. Morris for mouse breeding, genotyping, and viral injection support; S. Michael and A. Hu for histology support; M. Ramirez and B. Zemelman for rAAV production; H. Gainer for discussions about the oxytocin promoter; and E. Boyden for technical assistance.
Author information
Authors and Affiliations
Contributions
D.A., J.N.B. and S.M.S. designed the experiments and analysed data. D.A. and J.N.B. performed experiments. H.H.S. performed molecular cloning for viral constructs. S.M.S. and D.A. wrote the manuscript with comments from all of the authors.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Information
This file contains Supplementary Figures 1-16 and Supplementary Table 1. (PDF 4661 kb)
Rights and permissions
About this article
Cite this article
Atasoy, D., Betley, J., Su, H. et al. Deconstruction of a neural circuit for hunger. Nature 488, 172–177 (2012). https://doi.org/10.1038/nature11270
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nature11270
This article is cited by
-
AgRP neurons encode circadian feeding time
Nature Neuroscience (2024)
-
Neuroactive steroids in the neuroendocrine control of food intake, metabolism, and reproduction
Endocrine (2024)
-
Reduction of oxytocin-containing neurons and enhanced glymphatic activity in the hypothalamic paraventricular nucleus of patients with type 2 diabetes mellitus
Acta Neuropathologica Communications (2023)
-
Programming of metabolism by adipokines during development
Nature Reviews Endocrinology (2023)
-
Advanced neurobiological tools to interrogate metabolism
Nature Reviews Endocrinology (2023)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.