Abstract
Preclinical studies of human immunodeficiency virus type 1 (HIV-1) vaccine candidates have typically shown post-infection virological control, but protection against acquisition of infection has previously only been reported against neutralization-sensitive virus challenges1,2,3. Here we demonstrate vaccine protection against acquisition of fully heterologous, neutralization-resistant simian immunodeficiency virus (SIV) challenges in rhesus monkeys. Adenovirus/poxvirus and adenovirus/adenovirus-vector-based vaccines expressing SIVSME543 Gag, Pol and Env antigens resulted in an 80% or greater reduction in the per-exposure probability of infection4,5 against repetitive, intrarectal SIVMAC251 challenges in rhesus monkeys. Protection against acquisition of infection showed distinct immunological correlates compared with post-infection virological control and required the inclusion of Env in the vaccine regimen. These data demonstrate the proof-of-concept that optimized HIV-1 vaccine candidates can block acquisition of stringent, heterologous, neutralization-resistant virus challenges in rhesus monkeys.
Similar content being viewed by others
Main
Despite the recent demonstration of partial HIV-1 vaccine efficacy in humans6, the immune responses required to protect against acquisition of infection have remained unclear. Preclinical studies of HIV-1 vaccine candidates have begun to elucidate immunological correlates of protection against neutralization-sensitive viruses1,2,3, but no study has to date reported vaccine protection against acquisition of heterologous, neutralization-resistant virus challenges1,7,8. Mucosal SIVMAC251 infection of rhesus monkeys represents a stringent preclinical model of a highly pathogenic, neutralization-resistant virus swarm1,9,10, and repetitive mucosal challenges more closely mimic sexual HIV-1 transmission in humans than do single high-dose challenges10. We therefore performed two studies to evaluate the protective efficacy of optimized adenovirus/poxvirus and adenovirus/adenovirus vector-based vaccines against repetitive, heterologous, intrarectal SIVMAC251 challenges in rhesus monkeys.
In the first study, 40 Indian-origin rhesus monkeys (Macaca mulatta) that did not express the class I alleles Mamu-A*01, Mamu-B*08, and Mamu-B*17 associated with spontaneous virological control11,12,13 were immunized by the intramuscular route with the following vaccine regimens expressing SIVSME543 Gag-Pol and Env immunogens (N = 8 per group): (1) DNA prime, modified vaccinia Ankara (MVA) boost; (2) MVA prime, MVA boost; (3) adenovirus serotype 26 (Ad26) prime, MVA boost; (4) MVA prime, Ad26 boost; and (5) sham controls. Groups were balanced for susceptible and resistant TRIM5α alleles1,14. Monkeys were primed once at week 0 with 2 × 1010 viral particles of Ad26 vectors or 108 plaque-forming units (p.f.u.) of MVA vectors, or three times at weeks 0, 4, and 8 with 5 mg of DNA vaccines. Animals were then boosted once at week 24 with 2 × 1010 viral particles of Ad26 vectors or 108 p.f.u. of MVA vectors.
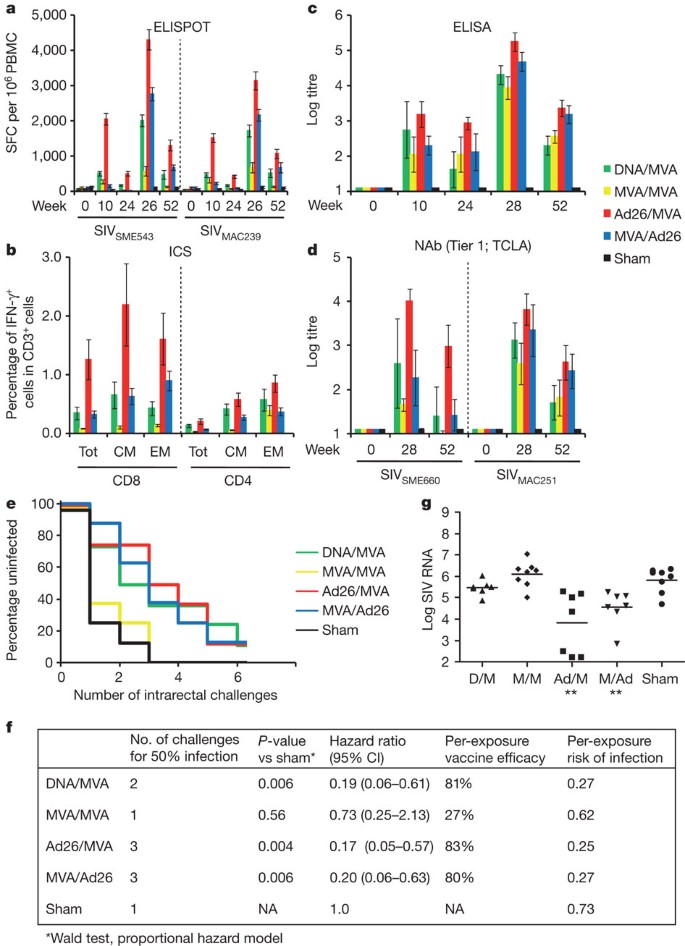
The vaccine regimens elicited distinct profiles of cellular and humoral immune responses, as measured by IFN-γ ELISPOT assays (Fig. 1a and Supplementary Fig. 1), multiparameter intracellular cytokine staining (ICS) assays8,15,16,17 (Fig. 1b and Supplementary Fig. 2), cellular immune breadth (Supplementary Fig. 3), SIVMAC251 Env-specific binding antibody ELISAs (Fig. 1c), tier 1 neutralizing antibody (NAb) assays against tissue culture laboratory adapted (TCLA) tier 1 SIVSME660 (CP3C-P-A8) and SIVMAC251 (TCLA) pseudoviruses (Fig. 1d), and antibody-dependent cellular cytotoxicity (ADCC) and antibody-dependent cell-mediated virus inhibition (ADCVI) assays (Supplementary Fig. 4). Tier 2 NAb responses against neutralization-resistant SIVSME660 (CR54-PK-2A5) and SIVMAC251 (SIVMAC251.30) pseudoviruses, however, were below the 50% neutralization cutoff for positivity, although positive trends were observed in all vaccinated groups (Supplementary Fig. 4).
a, Cellular immune responses to SIVSME543 and SIVMAC239 Gag, Pol and Env as determined by IFN-γ ELISPOT assays at weeks 0, 10, 24, 26, and 52. PBMC, peripheral blood mononuclear cells; SFC, spot-forming cells. b, CD8+ and CD4+ total, central/transitional memory (CM; CD28+CD95+), and effector memory (EM; CD28−CD95+) responses to Gag, Pol and Env as determined by multiparameter IFN-γ ICS assays at week 26. c, SIVMAC251 Env ELISAs at weeks 0, 10, 24, 28, and 52. d, SIVSME660 and SIVMAC251 tier 1 pseudovirus NAb assays at weeks 0, 28, and 52. Error bars represent s.e.m. e, Number of challenges required for acquisition of infection in each vaccine group. f, Statistical analyses include the number of challenges required for 50% infection, hazard ratios with 95% confidence intervals (CI), per-exposure vaccine efficacy and per-exposure risks of infection in each group. P-values reflect Wald tests using a proportional hazard model. g, Log SIV RNA copies per ml are depicted for each vaccine group at viral set point (day 84). **P = 0.0037, Wilcoxon rank-sum tests. The horizontal lines represent mean set point log viral loads. Ad, adenovirus; D, DNA; M, MVA.
To evaluate the protective efficacy of these vaccine regimens, all monkeys were challenged repetitively beginning at week 52 (six months following the boost immunization) with six intrarectal inoculations of the heterologous virus SIVMAC251 using a 1:1,000 dilution (930 half-maximal tissue-culture infectious dose (TCID50)) of our challenge stock9. After the first challenge, 75% of sham control monkeys became infected, compared with only 12–25% of the animals that received the heterologous vector regimens DNA/MVA, Ad26/MVA, and MVA/Ad26 (Fig. 1e). The percentage of uninfected animals declined proportionately with each challenge, and the majority of vaccinees and all controls were infected by the end of the challenge protocol. Monkeys that received the Ad26/MVA and MVA/Ad26 vaccines required three challenges to infect 50% of animals in each group, whereas only one challenge was required to infect 50% of animals in the control group (P = 0.004 and P = 0.006, respectively, Wald tests, proportional hazard model). The heterologous vector regimens also showed decreased hazard ratios of 0.17 (95% confidence interval (CI) 0.05–0.57) to 0.20 (CI 0.06–0.63) compared with the controls, corresponding to an 80–83% reduction in the per-exposure probability of infection (Fig. 1f; vaccine efficacy VE = 1 – hazard ratio), as previously described4,5. These data demonstrate vaccine protection against acquisition of infection following repetitive, heterologous, intrarectal SIVMAC251 challenges.
Control monkeys showed peak viral loads on day 14 following infection and then relatively stable mean set point viral loads of 5.85 log SIV RNA copies per millilitre (Supplementary Fig. 5). The Ad26/MVA and the MVA/Ad26 vaccines resulted, respectively, in at least 2.32 and 1.08 log reductions of mean set point viral loads compared with sham controls for over 250 days (P = 0.0037 for each vaccine versus sham, Wilcoxon rank-sum tests) (Fig. 1g and Supplementary Fig. 5). Moreover, half the animals in the Ad26/MVA group either demonstrated rapid and durable virological control to undetectable levels (Fig. 1g; N = 3) or remained uninfected (Fig. 1e; N = 1). The Ad26/MVA and MVA/Ad26 vaccines also afforded a survival advantage as compared with the controls (P = 0.025, log-rank test) (Supplementary Fig. 6).
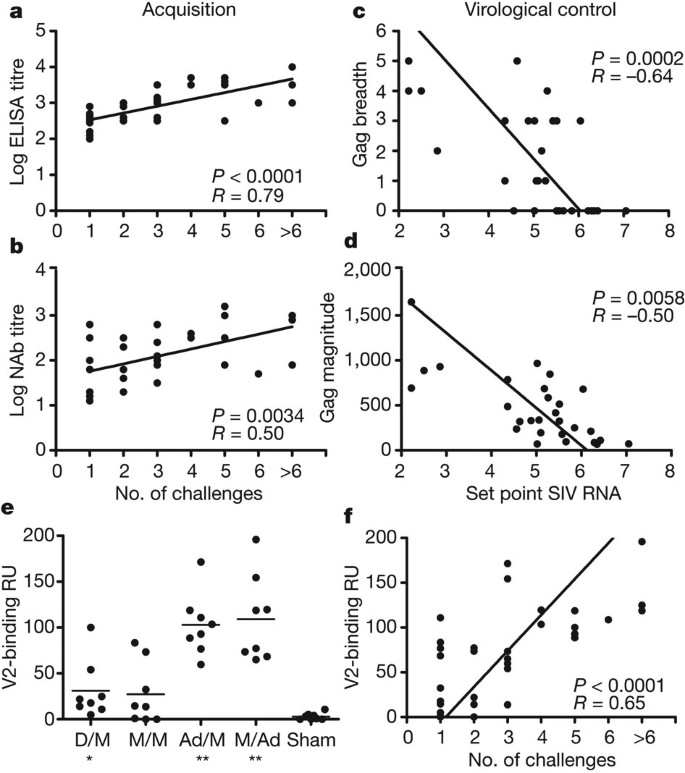
We next evaluated the immunological correlates of protection against acquisition of infection, defined as the number of challenges required to establish infection, and virological control, defined as set point viral loads. Our pre-specified primary immunological correlates analysis (Supplementary Table 1) demonstrated that protection against acquisition of infection was best correlated with Env-specific binding ELISA antibody responses (Fig. 2a; P < 0.0001, Spearman rank-correlation test) and tier 1 NAb titres (Fig. 2b; P = 0.0034) immediately before challenge. Protection against acquisition of infection also correlated with V2-specific antibodies that presumably represented a subset of total Env-specific binding antibodies (Fig. 2e, f; P < 0.0001). Virological control was correlated with Gag ELISPOT breadth (Fig. 2c; P = 0.0002) and magnitude (Fig. 2d; P = 0.0058) immediately before challenge, consistent with our previous observations18.
a, b, Correlation of log ELISA titres immediately before challenge (a) and log tier 1 NAb titres immediately before challenge (b) with the number of challenges required to establish infection. c, d, Correlation of Gag ELISPOT breadth before challenge (c) and Gag ELISPOT magnitude before challenge (d) with set point viral loads following challenge. Correlates analyses included the 32 vaccinated monkeys (a, b) or the 29 vaccinated animals that became infected (c, d) and did not include the sham controls. P-values reflect Spearman rank-correlation tests. e, V2-specific binding antibodies assessed by surface plasmon resonance response units (RU) for each vaccine group at week 30. *P = 0.002, **P = 0.0007, Wilcoxon rank-sum tests. The horizontal lines represent mean responses. f, Correlation of V2-specific antibody responses with the number of challenges required to establish infection.
In our exploratory immunological correlates analysis, we evaluated 35 humoral and cellular immune parameters at both peak and memory time points before challenge as possible immunological correlates of acquisition and virological control following challenge. No additional immune parameters were significantly correlated with protection against acquisition of infection in this analysis after multiple comparison adjustments (Supplementary Table 2). Gag-, Pol- and Env-specific effector memory CD8+ T-lymphocyte responses exhibited trends towards protection against acquisition, but did not achieve statistical significance according to our pre-specified criteria. In contrast, multiple humoral and cellular immune responses were significantly correlated with virological control (Supplementary Table 3), including Env ELISA, NAb and ADCC responses as well as Gag ELISPOT magnitude and breadth, Pol ELISPOT magnitude and Env CD4+ effector memory responses. These data support a model in which protection against acquisition of infection is correlated with vaccine-elicited Env antibody responses, whereas virological control is correlated with both T-lymphocyte and antibody responses. These distinct immunological correlates probably reflect fundamentally different biologic requirements for blocking establishment of infection at the mucosal site of inoculation compared with controlling viral replication after infection has become disseminated19. However, the actual mechanisms of protection remain to be determined.
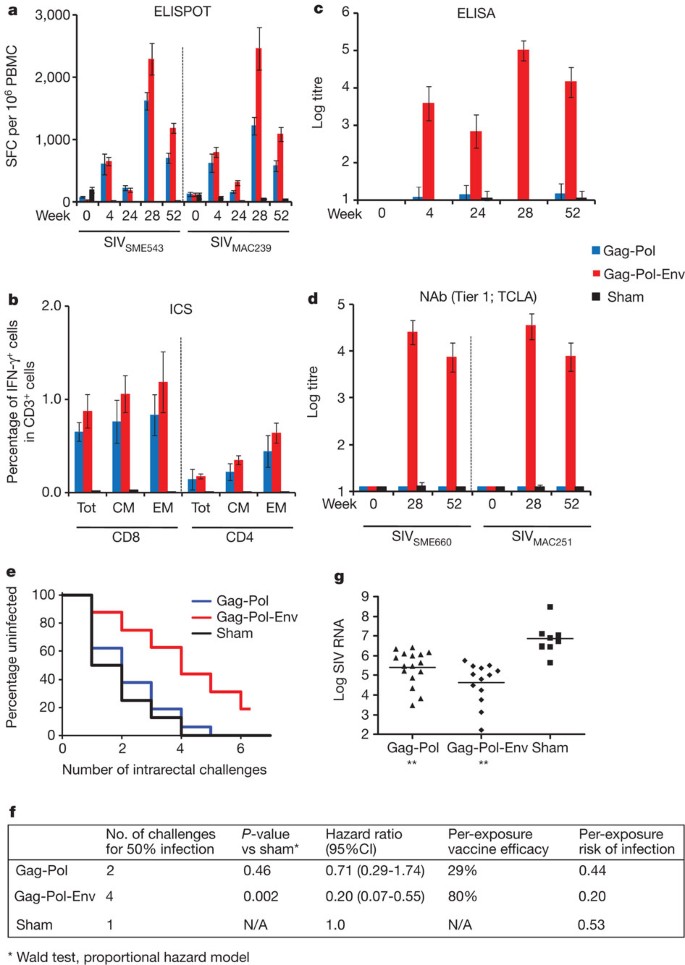
We next evaluated directly the hypothesis that Env was critical for blocking acquisition of infection in this system. In the second study, 40 rhesus monkeys that did not express the class I alleles Mamu-A*01, Mamu-B*08 and Mamu-B*17 were immunized by the intramuscular route with Ad35 prime20, Ad26 boost21 vaccine regimens expressing (1) SIVSME543 Gag-Pol (N = 16), (2) SIVSME543 Gag-Pol and Env (N = 16) and (3) sham controls (N = 8). Groups were balanced for susceptible and resistant TRIM5α alleles1,14. Monkeys were primed once at week 0 with 2 × 1010 viral particles of Ad35 vectors and boosted once at week 24 with 2 × 1010 viral particles of Ad26 vectors. Cellular immune responses were assessed by IFN-γ ELISPOT assays (Fig. 3a and Supplementary Fig. 7) and multiparameter ICS assays in both the periphery (Fig. 3b and Supplementary Fig. 8) and in colorectal mucosa (Supplementary Fig. 9). Env-specific humoral immune responses were assessed by ELISAs in both the periphery (Fig. 3c) and in colorectal mucosa (Supplementary Fig. 10), tier 1 NAb assays (Fig. 3d) and ADCC assays (Supplementary Fig. 11). Only marginal tier 2 NAb responses were observed (Supplementary Fig. 11).
a, Cellular immune responses to SIVSME543 and SIVMAC239 Gag, Pol and Env as determined by IFN-γ ELISPOT assays at weeks 0, 4, 24, 28 and 52. b, CD8+ and CD4+ total, central/transitional memory (CM; CD28+CD95+), and effector memory (EM; CD28−CD95+) responses to Gag, Pol and Env as determined by multiparameter IFN-γ ICS assays at week 28. c, SIVMAC251 Env ELISAs at weeks 0, 4, 24, 28 and 52. d, SIVSME660 and SIVMAC251 tier 1 pseudovirus NAb assays at weeks 0, 28 and 52. Error bars represent s.e.m. e, Number of challenges required for acquisition of infection in each vaccine group. f, Statistical analyses include the number of challenges required for 50% infection, hazard ratios with 95% confidence intervals (CI), per-exposure vaccine efficacy, and per-exposure risks of infection in each group. P-values reflect Wald tests using a proportional hazard model. g, Log SIV RNA copies per ml are depicted for each vaccine group at viral set point (day 84). **P < 0.001, Wilcoxon rank-sum tests. The horizontal lines reflect mean set point log viral loads.
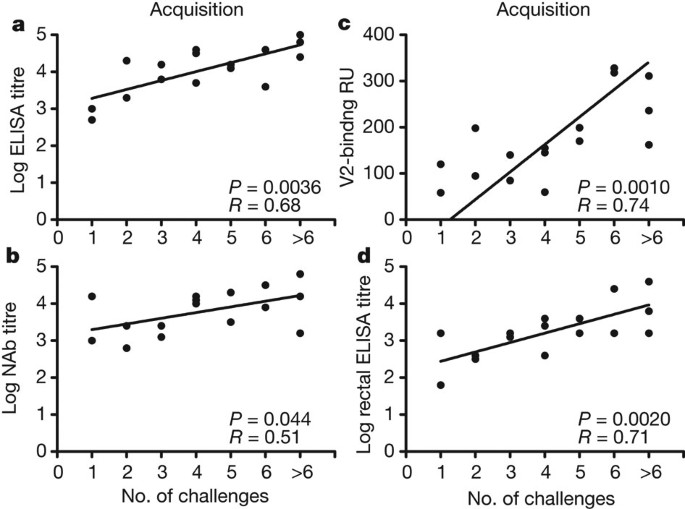
We assessed protective efficacy of these vaccine regimens against repetitive, heterologous, intrarectal SIVMAC251 challenges as described in the first study. After the first challenge, 50% of sham control monkeys became infected, compared with only 12% of the animals that received the Gag-Pol-Env vaccine (Fig. 3e). The monkeys that received the Gag-Pol-Env vaccine required four challenges to infect 50% of animals in each group, whereas only one challenge was required to infect 50% of animals in the control group (Fig. 3f; P = 0.002, Wald test, proportional hazard model). Moreover, the Gag-Pol-Env vaccine resulted in a decreased hazard ratio of 0.20 (CI 0.07–0.55), corresponding to an 80% reduction in the per-exposure probability of infection. In contrast, the Gag-Pol vaccine afforded only a marginal protective effect, demonstrating the critical role of Env in blocking acquisition of infection in this model. The Gag-Pol and Gag-Pol-Env vaccines resulted in, respectively, at least 1.59 log and 2.18 log reductions of set point viral loads compared with controls (Fig. 3g and Supplementary Fig. 12; P = 0.0006 and 0.0002, respectively, Wilcoxon rank-sum tests). Immunological correlates of protection against acquisition of infection were consistent with the first study, and both peripheral (Fig. 4a–c) and rectal (Fig. 4d) Env-specific IgG correlated with reduced acquisition risk.
a–d, Correlation of log ELISA titres immediately before challenge (a), log tier 1 NAb titres immediately before challenge (b), V2-specific antibody responses (c) and rectal IgG antibody responses (d) with the number of challenges required to establish infection. Correlates analyses included the 16 Gag-Pol-Env-vaccinated monkeys and did not include the Gag-Pol-vaccinated monkeys or the sham controls. P-values reflect Spearman rank-correlation tests.
Taken together, these data demonstrate that optimized adenovirus/poxvirus and adenovirus/adenovirus vector-based vaccines afforded significant protection against acquisition of infection following highly pathogenic, heterologous, neutralization-resistant SIVMAC251 challenges in rhesus monkeys (Figs 1e, 3e and Supplementary Fig. 13). Although several studies have previously shown partial protection against acquisition of neutralization-sensitive virus challenges1,2,3, no HIV-1 vaccine candidate has to date blocked acquisition of heterologous, difficult-to-neutralize virus challenges, including Ad5 (ref. 7), DNA/Ad5 (ref. 1) and cytomegalovirus8 vaccines. In particular, a recent study demonstrated that a DNA/Ad5 vaccine afforded partial protection against acquisition of SIVSME660, which is a neutralization-sensitive tier 1A virus in TZM-bl neutralization assays, but the same vaccine afforded no efficacy against neutralization-resistant SIVMAC251 (ref. 1), highlighting important differences in the stringencies between these two SIV challenge models as well as potentially important phenotypic differences between adenovirus serotypes17. However, we note that the acquisition effect in the present study was relative rather than absolute, and that the majority of vaccinees became infected by the end of the challenge protocol.
Our studies also demonstrate that inclusion of Env in the vaccine was required for the acquisition effect (Fig. 3e), despite an 18% difference in the Env amino acid sequences between the vaccine strain and challenge virus. Moreover, our immunological correlates analyses (Figs 2, 4 and Supplementary Tables 1–3) suggest that Env-specific antibodies are critical for blocking acquisition of infection, whereas multiple cellular and humoral immune responses correlate with virological control, although the actual mechanisms of protection remain to be determined. In addition, the RV144 immunological correlates analyses raised the hypothesis that vaccine-elicited V1/V2-specific antibodies may reduce HIV-1 acquisition risk in humans22. Our data (Figs 2f, 4c) are consistent with this hypothesis, although it remains unclear whether V2-specific antibodies actually protect or simply represent a marker for other Env-specific antibodies or other protective factors.
Considerable efforts are currently underway to identify and to reverse engineer potent, broadly reactive monoclonal antibodies23,24. Although the induction of such NAb responses by a vaccine would presumably be highly desirable, no Env immunogens have to date been developed that can elicit these responses. Our findings suggest that a substantial degree of protection can be achieved against stringent virus challenges even in the absence of high titres of tier 2 NAbs, perhaps reflecting the importance of antibody effector functions that may not be fully measured by traditional virus neutralization assays. Of note, the partial protection in the present study was observed with vectored Env and without a purified Env protein subunit boost. The degree to which an Env protein boost may further improve the protective efficacy afforded by these vaccine regimens remains to be determined.
In summary, our data demonstrate the proof-of-concept that vaccination can protect against acquisition of stringent, heterologous, neutralization-resistant SIVMAC251 challenges in rhesus monkeys. These findings, together with the observations of a critical requirement for Env and the distinct immunological correlates of protection against acquisition of infection and virological control, pave novel paths forward for HIV-1 vaccine development.
Methods Summary
For each study, 40 Indian-origin rhesus monkeys (Macaca mulatta) were vaccinated with DNA, MVA25, Ad26 (ref. 21) or Ad35 (ref. 20) expressing SIVSME543 Gag-Pol and/or Env antigens or received a sham vaccine. Cellular immune responses were measured by IFN-γ ELISPOT18, multiparameter intracellular cytokine staining (ICS)8,15,16,17 and epitope mapping26 assays. Humoral immune responses were measured by Env ELISA27, TZM-bl pseudovirus neutralizing antibody (NAb)28, antibody-dependent cellular cytotoxicity (ADCC)29 and antibody-dependent cell-mediated virus inhibition (ADCVI)30 assays. Six months after the boost immunization, all monkeys received six challenges by the intrarectal route with the fully heterologous, neutralization-resistant virus SIVMAC251 using a 1:1,000 dilution (930 TCID50) of our challenge stock9. Protective efficacy was determined by resistance to acquisition of infection, defined as the number of challenges required to establish infection, and virological control, defined as set point viral loads. Immunological correlates of protection against acquisition of infection and virological control were evaluated by pre-specified primary and exploratory analyses.
Online Methods
Animals, immunizations and challenges
Indian-origin, outbred, young adult, male and female, specific pathogen-free (SPF) rhesus monkeys (Macaca mulatta, 80 animals) that did not express the class I alleles Mamu-A*01, Mamu-B*08 and Mamu-B*17 associated with spontaneous virological control11,12,13 were housed at the New England Primate Research Center (NEPRC), Southborough, Massachusetts, USA. A total of 40 animals was used for each study. Groups were balanced for susceptible and resistant TRIM5α alleles1,14. Immunizations were performed by the intramuscular route in the quadriceps muscles with 2 × 1010 viral particles of Ad35 vectors20, 2 × 1010 viral particles of Ad26 vectors21, 108 p.f.u. of MVA vectors25, or 5 mg of DNA vaccines expressing SIVSME543 Gag-Pol and/or Env gp140. Monkeys were primed at week 0 and boosted at week 24, except DNA vaccine priming that was performed at weeks 0, 4 and 8. To evaluate for protective efficacy and immunological correlates, all monkeys were challenged repetitively beginning at week 52 with six intrarectal inoculations of the heterologous virus SIVMAC251 using a 1:1,000 dilution (930 TCID50) of our challenge stock9. Monkeys were bled weekly for viral loads (Siemans Diagnostics), and the date of infection was defined as the last challenge time point before the first positive SIV RNA level. Animals were followed to determine set point viral loads. All animal studies were approved by the Harvard Medical School Institutional Animal Care and Use Committee (IACUC).
Cellular immune assays
SIV-specific cellular immune responses were assessed by IFN-γ ELISPOT assays18 and multiparameter intracellular cytokine staining (ICS) assays8,15,16,17 essentially as described. ELISPOT assays used pools of SIVSME543 and SIVMAC239 Gag, Pol and Env peptides. Analyses of cellular immune breadth used sub-pools of 10 peptides covering each antigen. Peptides were 15 amino acids in length and overlapped by 11 amino acids. Nine-colour ICS assays used monoclonal antibodies (Becton Dickinson) against CD3 (SP34; Alexa700), CD4 (L200; AmCyan), CD8 (SK1; allophycocyanin-cyanine7 (APC-Cy7)), CD28 (L293; peridinin chlorophyll-A-cyanine5.5 (PerCP-Cy5.5)), CD95 (DX2; phycoerythrin (PE)), CD69 (TP1.55.3; phycoerythrin-Texas Red (energy-coupled dye; ECD); Beckman Coulter), IFN-γ (B27; phycoerythrin-cyanine7 (PE-Cy7)), IL-2 (MQ1-17H12; allophycocyanin (APC)) and TNF-α (Mab11; fluorescein isothiocyanate (FITC)). IFN-γ backgrounds were consistently <0.01% in peripheral blood mononuclear cells and <0.05% in colorectal biopsy specimens.
Humoral immune assays
SIV-specific humoral immune responses were assessed by SIVMAC251 Env ELISAs27, TZM-bl luciferase-based virus neutralization assays28 against tier 1 SIVSME660 (CP3C-P-A8) and SIVMAC251 (TCLA) pseudoviruses, TZM-bl virus neutralization assays against tier 2 SIVSME660 (CR54-PK-2A5) and SIVMAC251 (SIVMAC251.30) pseudoviruses, antibody-dependent cellular cytotoxicity (ADCC) assays29, and antibody-dependent cell-mediated virus inhibition (ADCVI) assays30. V2-binding assays were performed by surface plasmon resonance with a Biacore 2000 or T200 using a 1:50 serum dilution and a cyclic SIVSME543 V2 peptide containing an amino-terminal biotin tag (CIKNNSCAGLEQEPMIGCKFNMTGLKRDKKIEYNETWYSRDLICEQPANGSESKCY) and immobilized on streptavidin-coated CM5 chips. Mucosal antibodies were assessed using rectal secretions collected with Weck-Cel sponges. Approximately 100 μl rectal secretions were eluted and diluted sixfold, and total IgG and IgA as well as SIV Env-specific IgG and IgA (Immune Technology Corporation) were measured by ELISA using a biotin-conjugated anti-monkey IgG and IgA (Alpha Diagnostics) secondary antibody. Mucosal titres were defined as endpoint ELISA titres multiplied by the dilution of the eluted secretions. Samples showed comparable levels of total IgG.
Statistical analyses and immunological correlates
Protection against acquisition of infection was analysed using Wald tests with a proportional hazard model and the exact conditional likelihood method for breaking ties. A discrete time model provided similar estimates. The number of challenges required for 50% infection of each group, hazard ratios with 95% confidence intervals, per-exposure vaccine efficacy and per-exposure risks of infection were quantified. Vaccine efficacy was defined as the reduction in the per-exposure probability of infection as previously described4,5. Analyses of virological and immunological data were performed by Wilcoxon rank-sum tests and analysis of survival by log-rank tests. For these tests, P < 0.05 was considered significant and two-tailed tests were performed. Immunological correlates were evaluated by a focused primary analysis and a detailed exploratory analysis using Spearman rank-correlation tests. In the primary analysis, P < 0.01 was considered significant, whereas in the exploratory analysis, P < 0.0014 was considered significant to adjust for multiple comparisons.
Change history
01 February 2012
Author initials for E.A.B. were corrected.
References
Letvin, N. L. et al. Immune and genetic correlates of vaccine protection against mucosal infection by SIV in monkeys. Sci. Transl. Med. 3, 81ra36 (2011)
Lai, L. et al. Prevention of infection by a granulocyte-macrophage colony-stimulating factor co-expressing DNA/modified vaccinia Ankara simian immunodeficiency virus vaccine. J. Infect. Dis. 204, 164–173 (2011)
Barnett, S. W. et al. Antibody-mediated protection against mucosal simian-human immunodeficiency virus challenge of macaques immunized with alphavirus replicon particles and boosted with trimeric envelope glycoprotein in MF59 adjuvant. J. Virol. 84, 5975–5985 (2010)
Hudgens, M. G. et al. Power to detect the effects of HIV vaccination in repeated low-dose challenge experiments. J. Infect. Dis. 200, 609–613 (2009)
Hudgens, M. G. & Gilbert, P. B. Assessing vaccine effects in repeated low-dose challenge experiments. Biometrics 65, 1223–1232 (2009)
Rerks-Ngarm, S. et al. Vaccination with ALVAC and AIDSVAX to prevent HIV-1 infection in Thailand. N. Engl. J. Med. 361, 2209–2220 (2009)
Wilson, N. A. et al. Vaccine-induced cellular immune responses reduce plasma viral concentrations after repeated low-dose challenge with pathogenic simian immunodeficiency virus SIVmac239. J. Virol. 80, 5875–5885 (2006)
Hansen, S. G. et al. Profound early control of highly pathogenic SIV by an effector memory T-cell vaccine. Nature 473, 523–527 (2011)
Liu, J. et al. Low-dose mucosal simian immunodeficiency virus infection restricts early replication kinetics and transmitted virus variants in rhesus monkeys. J. Virol. 84, 10406–10412 (2010)
Keele, B. F. et al. Low-dose rectal inoculation of rhesus macaques by SIVsmE660 or SIVmac251 recapitulates human mucosal infection by HIV-1. J. Exp. Med. 206, 1117–1134 (2009)
Yant, L. J. et al. The high-frequency major histocompatibility complex class I allele Mamu-B*17 is associated with control of simian immunodeficiency virus SIVmac239 replication. J. Virol. 80, 5074–5077 (2006)
Mothé, B. R. et al. Expression of the major histocompatibility complex class I molecule Mamu-A*01 is associated with control of simian immunodeficiency virus SIVmac239 replication. J. Virol. 77, 2736–2740 (2003)
Loffredo, J. T. et al. Mamu-B*08-positive macaques control simian immunodeficiency virus replication. J. Virol. 81, 8827–8832 (2007)
Lim, S. Y. et al. TRIM5α modulates immunodeficiency virus control in rhesus monkeys. PLoS Pathog. 6, e1000738 (2010)
Okoye, A. et al. Progressive CD4+ central memory T cell decline results in CD4+ effector memory insufficiency and overt disease in chronic SIV infection. J. Exp. Med. 204, 2171–2185 (2007)
Pitcher, C. J. et al. Development and homeostasis of T cell memory in rhesus macaque. J. Immunol. 168, 29–43 (2002)
Liu, J. et al. Magnitude and phenotype of cellular immune responses elicited by recombinant adenovirus vectors and heterologous prime-boost regimens in rhesus monkeys. J. Virol. 82, 4844–4852 (2008)
Liu, J. et al. Immune control of an SIV challenge by a T-cell-based vaccine in rhesus monkeys. Nature 457, 87–91 (2009)
Haase, A. T. Targeting early infection to prevent HIV-1 mucosal transmission. Nature 464, 217–223 (2010)
Vogels, R. et al. Replication-deficient human adenovirus type 35 vectors for gene transfer and vaccination: efficient human cell infection and bypass of preexisting adenovirus immunity. J. Virol. 77, 8263–8271 (2003)
Abbink, P. et al. Comparative seroprevalence and immunogenicity of six rare serotype recombinant adenovirus vaccine vectors from subgroups B and D. J. Virol. 81, 4654–4663 (2007)
Haynes, B. Case control study of the RV144 trial for immune correlates: the analysis and way forward. AIDS Vaccine Conference 2011 (Bangkok, Thailand, 2011)
Walker, L. M. et al. Broad and potent neutralizing antibodies from an African donor reveal a new HIV-1 vaccine target. Science 326, 285–289 (2009)
Wu, X. et al. Rational design of envelope identifies broadly neutralizing human monoclonal antibodies to HIV-1. Science 329, 856–861 (2010)
Ourmanov, I. et al. Comparative efficacy of recombinant modified vaccinia virus Ankara expressing simian immunodeficiency virus (SIV) Gag-Pol and/or Env in macaques challenged with pathogenic SIV. J. Virol. 74, 2740–2751 (2000)
Barouch, D. H. et al. Mosaic HIV-1 vaccines expand the breadth and depth of cellular immune responses in rhesus monkeys. Nature Med. 16, 319–323 (2010)
Nkolola, J. P. et al. Breadth of neutralizing antibodies elicited by stable, homogeneous clade A and clade C HIV-1 gp140 envelope trimers in guinea pigs. J. Virol. 84, 3270–3279 (2010)
Montefiori, D. C. Evaluating neutralizing antibodies against HIV, SIV and SHIV in luciferase reporter gene assays. Curr. Protoc. Immunol. 12, Unit 12.11. (2004)
Ferrari, G. et al. An HIV-1 gp120 envelope human monoclonal antibody that recognizes a C1 conformational epitope mediates potent antibody-dependent cellular cytotoxicity (ADCC) activity and defines a common ADCC epitope in human HIV-1 serum. J. Virol. 85, 7029–7036 (2011)
Forthal, D. N., Landucci, G. & Daar, E. S. Antibody from patients with acute human immunodeficiency virus (HIV) infection inhibits primary strains of HIV type 1 in the presence of natural-killer effector cells. J. Virol. 75, 6953–6961 (2001)
Acknowledgements
We thank M. Pensiero, A. Fauci, E. Borducchi, S. Clark, R. Hamel, S. King, P. Kozlowski, A. La Porte, G. Landucci, A. Oza, J. Perry, L. Peter, A. Riggs, G. Shaw, N. Simmons, K. Smith, K. Stanley, F. Stephens, Y.-H. Sun, G. Weverling and E. Zablowsky for advice, assistance and reagents. We thank L. Picker, G. Silvestri and B. Walker for critically reviewing this manuscript. The SIVMAC239 peptide pools were obtained from the NIH AIDS Research and Reference Reagent Program. We acknowledge support from the US Military Research and Material Command and the US Military HIV Research Program (W81XWH-07-2-0067); the Ragon Institute of MGH, MIT and Harvard; the National Institutes of Health (AI066924, AI078526, AI084794, AI095985, AI060354, AI002642, RR000168); and the Bill and Melinda Gates Foundation. The opinions in this manuscript are those of the authors and do not reflect the views of the US Department of Defense.
Author information
Authors and Affiliations
Contributions
D.H.B., M.G.P., H.S., J.C.S., J.G., M.L.R., J.H.K., M.A.M. and N.L.M. designed the study and analysed data. J.L., H.L., D.M.L., M.J.I. and A.S. performed the cellular immunogenicity assays. H.L., M.J.I., M.S.S., G.F., D.N.F., E.A.B. and M.R. performed the humoral immunogenicity assays. L.F.M., P.A., I.O. and V.M.H. prepared the vaccine constructs. A.C. and K.G.M. led the clinical care of the rhesus monkeys. D.S. led the statistical analyses. D.H.B. led the study and wrote the paper with all co-authors.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Information
The file contains Supplementary Tables 1-3 and Supplementary Figures 1-13 with legends. (PDF 636 kb)
Rights and permissions
About this article
Cite this article
Barouch, D., Liu, J., Li, H. et al. Vaccine protection against acquisition of neutralization-resistant SIV challenges in rhesus monkeys. Nature 482, 89–93 (2012). https://doi.org/10.1038/nature10766
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nature10766
This article is cited by
-
Immunogenic arenavirus vector SIV vaccine reduces setpoint viral load in SIV-challenged rhesus monkeys
npj Vaccines (2023)
-
Differential V2-directed antibody responses in non-human primates infected with SHIVs or immunized with diverse HIV vaccines
Nature Communications (2022)
-
Impact of adjuvants on the biophysical and functional characteristics of HIV vaccine-elicited antibodies in humans
npj Vaccines (2022)
-
Functional analysis of a monoclonal antibody reactive against the C1C2 of Env obtained from a patient infected with HIV-1 CRF02_AG
Retrovirology (2021)
-
A recombinant measles virus vaccine strongly reduces SHIV viremia and virus reservoir establishment in macaques
npj Vaccines (2021)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.