Abstract
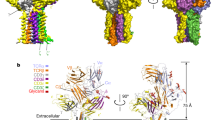
The pre-T-cell antigen receptor (pre-TCR), expressed by immature thymocytes, has a pivotal role in early T-cell development, including TCR β-selection, survival and proliferation of CD4−CD8− double-negative thymocytes, and subsequent αβ T-cell lineage differentiation1,2,3. Whereas αβTCR ligation by the peptide-loaded major histocompatibility complex initiates T-cell signalling4, pre-TCR-induced signalling occurs by means of a ligand-independent dimerization event5. The pre-TCR comprises an invariant α-chain (pre-Tα) that pairs with any TCR β-chain (TCRβ) following successful TCR β-gene rearrangement6. Here we provide the basis of pre-Tα–TCRβ assembly and pre-TCR dimerization. The pre-Tα chain comprised a single immunoglobulin-like domain that is structurally distinct from the constant (C) domain of the TCR α-chain7; nevertheless, the mode of association between pre-Tα and TCRβ mirrored that mediated by the Cα–Cβ domains of the αβTCR. The pre-TCR had a propensity to dimerize in solution, and the molecular envelope of the pre-TCR dimer correlated well with the observed head-to-tail pre-TCR dimer. This mode of pre-TCR dimerization enabled the pre-Tα domain to interact with the variable (V) β domain through residues that are highly conserved across the Vβ and joining (J) β gene families, thus mimicking the interactions at the core of the αβTCR’s Vα–Vβ interface. Disruption of this pre-Tα–Vβ dimer interface abrogated pre-TCR dimerization in solution and impaired pre-TCR expression on the cell surface. Accordingly, we provide a mechanism of pre-TCR self-association that allows the pre-Tα chain to simultaneously ‘sample’ the correct folding of both the V and C domains of any TCR β-chain, regardless of its ultimate specificity, which represents a critical checkpoint in T-cell development. This unusual dual-chaperone-like sensing function of pre-Tα represents a unique mechanism in nature whereby developmental quality control regulates the expression and signalling of an integral membrane receptor complex.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
von Boehmer, H. Unique features of the pre-T-cell receptor α-chain: not just a surrogate. Nature Rev. Immunol. 5, 571–577 (2005)
Saint-Ruf, C. et al. Different initiation of pre-TCR and γδTCR signalling. Nature 406, 524–527 (2000)
Borowski, C., Li, X., Aifantis, I., Gounari, F. & von Boehmer, H. Pre-TCRα and TCRα are not interchangeable partners of TCRβ during T lymphocyte development. J. Exp. Med. 199, 607–615 (2004)
Zinkernagel, R. M. & Doherty, P. C. Restriction of in vitro T cell-mediated cytotoxicity in lymphocytic choriomeningitis within a syngeneic or semiallogeneic system. Nature 248, 701–702 (1974)
Yamasaki, S. et al. Mechanistic basis of pre-T cell receptor-mediated autonomous signaling critical for thymocyte development. Nature Immunol. 7, 67–75 (2005)
Saint-Ruf, C. Analysis and expression of a cloned pre-T cell receptor gene. Science 266, 1208–1212 (1994)
Garcia, K. C. et al. An αβ T cell receptor structure at 2.5 Å and its orientation in the TCR-MHC complex. Science 274, 209–219 (1996)
Kjer-Nielsen, L. et al. A structural basis for the selection of dominant αβ T cell receptors in antiviral immunity. Immunity 18, 53–64 (2003)
Kjer-Nielsen, L. et al. The 1.5 Å crystal structure of a highly selected antiviral T cell receptor provides evidence for a structural basis of immunodominance. Structure 10, 1521–1532 (2002)
Yamasaki, S. & Saito, T. Molecular basis for pre-TCR-mediated autonomous signaling. Trends Immunol. 28, 39–43 (2007)
Kuhns, M. S. & Davis, M. M. Disruption of extracellular interactions impairs T cell receptor-CD3 complex stability and signaling. Immunity 26, 357–369 (2007)
Beddoe, T. et al. Antigen ligation triggers a conformational change within the constant domain of the αβ T cell receptor. Immunity 30, 777–788 (2009)
van der Merwe, P. A. & Davis, S. J. Molecular interactions mediating T cell antigen recognition. Annu. Rev. Immunol. 21, 659–684 (2003)
Franke, D. & Svergun, D. I. DAMMIF, a program for rapid ab-initio shape determination in small-angle scattering. J. Appl. Crystallogr. 42, 342–346 (2009)
Svergun, D. I., Petoukhov, M. V. & Koch, M. H. Determination of domain structure of proteins from X-ray solution scattering. Biophys. J. 80, 2946–2953 (2001)
Dave, V. P. Hierarchical role of CD3 chains in thymocyte development. Immunol. Rev. 232, 22–33 (2009)
Allison, T. J., Winter, C. C., Fournie, J. J., Bonneville, M. & Garboczi, D. N. Structure of a human γδ T-cell antigen receptor. Nature 411, 820–824 (2001)
Melchers, F. The pre-B-cell receptor: selector of fitting immunoglobulin heavy chains for the B-cell repertoire. Nature Rev. Immunol. 5, 578–584 (2005)
Semenyuk, A. V. & Svergun, D. I. GNOM - a program package for small-angle scattering data processing. J. Appl. Crystallogr. 24, 537–540 (1991)
Brookes, E., Demeler, B., Rosano, C. & Rocco, M. The implementation of SOMO (SOlution MOdeller) in the UltraScan analytical ultracentrifugation data analysis suite: enhanced capabilities allow the reliable hydrodynamic modeling of virtually any kind of biomacromolecule. Eur. Biophys. J. 39, 423–435 (2010)
CCP4. The CCP4 suite: programs for protein crystallography. Acta Crystallogr. D 50, 760–763 (1994)
Adams, P. D. et al. PHENIX: building new software for automated crystallographic structure determination. Acta Crystallogr. D 58, 1948–1954 (2002)
Zwart, P. H. et al. Automated structure solution with the PHENIX suite. Methods Mol. Biol. 426, 419–435 (2008)
Emsley, P. & Cowtan, K. Coot: model-building tools for molecular graphics. Acta Crystallogr. D 60, 2126–2132 (2004)
Cole, J. L. et al. Analytical ultracentrifugation: sedimentation velocity and sedimentation equilibrium. Methods Cell Biol. 84, 143–179 (2008)
Schuck, P. Size-distribution analysis of macromolecules by sedimentation velocity ultracentrifugation and Lamm equation modeling. Biophys. J. 78, 1606–1619 (2000)
Rinner, O. et al. Identification of cross-linked peptides from large sequence databases. Nature Methods 5, 315–318 (2008)
Burger, R., Hansen-Hagge, T. E., Drexler, H. D. & Gramatzki, M. Heterogeneity of T-acute lymphoblastic leukemia (T-ALL) cell lines: suggestion for classification by immunophenotype and T-cell receptor studies. Leuk. Res. 23, 19–27 (1999)
Hirano, T. et al. In vitro immune response of human peripheral lymphocytes: IV. Specific induction of human suppressor T cells by an antiserum to the T leukemia cell line HSB. J. Immunol. 123, 1133–1140 (1979)
Holst, J., Vignali, K. M., Burton, A. R. & Vignali, D. A. A. Rapid analysis of T-cell selection in vivo using T cell-receptor retrogenic mice. Nature Methods 3, 191–197 (2006)
Acknowledgements
We thank S. Turner for discussions; S. Ramarathinam for technical assistance; the staff at the Australian synchrotron (MX and SAXS/WAXS beamlines) and Berkeley Advanced Light Source (SIBYLS beamline) for assistance with data collection; and T. Caradoc-Davies for advice on processing of merohedral twinned X-ray data. This research was supported by grants from the Australian Research Council and the National Health and Medical Research Council of Australia. A.W.P., T.T. and M.C.J.W. are supported by NHMRC Senior Research Fellowships, and D.I.G. is supported by an NHMRC Principal Research Fellowship. M.A.P. is supported by an ARC Future Fellowship and J.R. is supported by an ARC Federation Fellowship.
Author information
Authors and Affiliations
Contributions
S.S.P. solved the structure, undertook analysis, performed experiments and contributed to manuscript preparation. J.M., D.I.G. and J.R. contributed to the design and interpretation of experiments, project management and the writing of the manuscript. R.B., Z.C., L.K.-N., M.A.P., G.F.K., N.L.L.G., T.T. and A.W.P. performed experiments and contributed to the writing of the manuscript; C.W., S.H.C., N.K.W., T.B., N.P.C. and M.C.J.W. performed experiments. J.M. and J.R. were the joint senior authors—they co-led the investigation, devised the project, analysed the data and wrote the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Information
This file contains Supplementary Figures 1-8 with legends and Supplementary Tables 1-6. (PDF 4983 kb)
Rights and permissions
About this article
Cite this article
Pang, S., Berry, R., Chen, Z. et al. The structural basis for autonomous dimerization of the pre-T-cell antigen receptor. Nature 467, 844–848 (2010). https://doi.org/10.1038/nature09448
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nature09448
This article is cited by
-
Rapid cloning of antigen-specific T-cell receptors by leveraging the cis activation of T cells
Nature Biomedical Engineering (2022)
-
Bioinformatic analysis and functional predictions of selected regeneration-associated transcripts expressed by zebrafish microglia
BMC Genomics (2020)
-
NMR: an essential structural tool for integrative studies of T cell development, pMHC ligand recognition and TCR mechanobiology
Journal of Biomolecular NMR (2019)
-
Pre-TCRα supports CD3-dependent reactivation and expansion of TCRα-deficient primary human T-cells
Molecular Therapy - Methods & Clinical Development (2014)
-
Lysine-specific chemical cross-linking of protein complexes and identification of cross-linking sites using LC-MS/MS and the xQuest/xProphet software pipeline
Nature Protocols (2014)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.