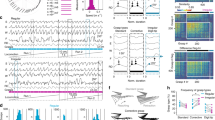
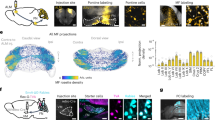
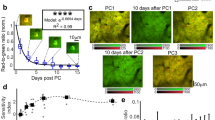
Abstract
Cortical neurons form specific circuits1, but the functional structure of this microarchitecture and its relation to behaviour are poorly understood. Two-photon calcium imaging can monitor activity of spatially defined neuronal ensembles in the mammalian cortex2,3,4,5. Here we applied this technique to the motor cortex of mice performing a choice behaviour. Head-fixed mice were trained to lick in response to one of two odours, and to withhold licking for the other odour6,7. Mice routinely showed significant learning within the first behavioural session and across sessions. Microstimulation8,9 and trans-synaptic tracing10,11 identified two non-overlapping candidate tongue motor cortical areas. Inactivating either area impaired voluntary licking. Imaging in layer 2/3 showed neurons with diverse response types in both areas. Activity in approximately half of the imaged neurons distinguished trial types associated with different actions. Many neurons showed modulation coinciding with or preceding the action, consistent with their involvement in motor control. Neurons with different response types were spatially intermingled. Nearby neurons (within ∼150 μm) showed pronounced coincident activity. These temporal correlations increased with learning within and across behavioural sessions, specifically for neuron pairs with similar response types. We propose that correlated activity in specific ensembles of functionally related neurons is a signature of learning-related circuit plasticity. Our findings reveal a fine-scale and dynamic organization of the frontal cortex that probably underlies flexible behaviour.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Song, S., Sjostrom, P. J., Reigl, M., Nelson, S. & Chklovskii, D. B. Highly nonrandom features of synaptic connectivity in local cortical circuits. PLoS Biol. 3, 1–13 (2005)
Stosiek, C., Garaschuk, O., Holthoff, K. & Konnerth, A. In vivo two-photon calcium imaging of neuronal networks. Proc. Natl Acad. Sci. USA 100, 7319–7324 (2003)
Kerr, J. N. et al. Spatial organization of neuronal population responses in layer 2/3 of rat barrel cortex. J. Neurosci. 27, 13316–13328 (2007)
Sato, T. R., Gray, N. W., Mainen, Z. F. & Svoboda, K. The functional microarchitecture of the mouse barrel cortex. PLoS Biol. 5, e189 (2007)
Dombeck, D. A., Khabbaz, A. N., Collman, F., Adelman, T. L. & Tank, D. W. Imaging large-scale neural activity with cellular resolution in awake, mobile mice. Neuron 56, 43–57 (2007)
Slotnick, B. & Restrepo, D. Olfactometry with mice. Curr. Protoc. Neurosci. Chapter 8, Unit–8.20 (2005)
Verhagen, J. V., Wesson, D. W., Netoff, T. I., White, J. A. & Wachowiak, M. Sniffing controls an adaptive filter of sensory input to the olfactory bulb. Nature Neurosci. 10, 631–639 (2007)
Li, C. X. & Waters, R. S. Organization of the mouse motor cortex studied by retrograde tracing and intracortical microstimulation (ICMS) mapping. Can. J. Neurol. Sci. 18, 28–38 (1991)
Ayling, O. G., Harrison, T. C., Boyd, J. D., Goroshkov, A. & Murphy, T. H. Automated light-based mapping of motor cortex by photoactivation of channelrhodopsin-2 transgenic mice. Nature Methods 6, 219–224 (2009)
Song, C. K., Enquist, L. W. & Bartness, T. J. New developments in tracing neural circuits with herpesviruses. Virus Res. 111, 235–249 (2005)
Fay, R. A. & Norgren, R. Identification of rat brainstem multisynaptic connections to the oral motor nuclei using pseudorabies virus. III. Lingual muscle motor systems. Brain Res. Brain Res. Rev. 25, 291–311 (1997)
Tanji, J. & Evarts, E. V. Anticipatory activity of motor cortex neurons in relation to direction of an intended movement. J. Neurophysiol. 39, 1062–1068 (1976)
Zohary, E., Shadlen, M. N. & Newsome, W. T. Correlated neuronal discharge rate and its implications for psychophysical performance. Nature 370, 140–143 (1994)
Travers, J. B., Dinardo, L. A. & Karimnamazi, H. Motor and premotor mechanisms of licking. Neurosci. Biobehav. Rev. 21, 631–647 (1997)
Rathelot, J. A. & Strick, P. L. Muscle representation in the macaque motor cortex: an anatomical perspective. Proc. Natl Acad. Sci. USA 103, 8257–8262 (2006)
Brecht, M. et al. Organization of rat vibrissa motor cortex and adjacent areas according to cytoarchitectonics, microstimulation, and intracellular stimulation of identified cells. J. Comp. Neurol. 479, 360–373 (2004)
Smith, B. N. et al. Pseudorabies virus expressing enhanced green fluorescent protein: A tool for in vitro electrophysiological analysis of transsynaptically labeled neurons in identified central nervous system circuits. Proc. Natl Acad. Sci. USA 97, 9264–9269 (2000)
O’Connor, D. H. et al. Vibrissa-based object localization in head-fixed mice. J. Neurosci. 30, 1947–1967 (2010)
Nimmerjahn, A., Kirchhoff, F., Kerr, J. N. & Helmchen, F. Sulforhodamine 101 as a specific marker of astroglia in the neocortex in vivo. Nature Methods 1, 31–37 (2004)
Kerr, J. N., Greenberg, D. & Helmchen, F. Imaging input and output of neocortical networks in vivo. Proc. Natl Acad. Sci. USA 102, 14063–14068 (2005)
Doucette, W. & Restrepo, D. Profound context-dependent plasticity of mitral cell responses in olfactory bulb. PLoS Biol. 6, e258 (2008)
Bair, W., Zohary, E. & Newsome, W. T. Correlated firing in macaque visual area MT: time scales and relationship to behavior. J. Neurosci. 21, 1676–1697 (2001)
Ts’o, D. Y., Gilbert, C. D. & Wiesel, T. N. Relationships between horizontal interactions and functional architecture in cat striate cortex as revealed by cross-correlation analysis. J. Neurosci. 6, 1160–1170 (1986)
Georgopoulos, A. P., Taira, M. & Lukashin, A. Cognitive neurophysiology of the motor cortex. Science 260, 47–52 (1993)
Fujisawa, S., Amarasingham, A., Harrison, M. T. & Buzsaki, G. Behavior-dependent short-term assembly dynamics in the medial prefrontal cortex. Nature Neurosci. 11, 823–833 (2008)
Constantinidis, C., Franowicz, M. N. & Goldman-Rakic, P. S. Coding specificity in cortical microcircuits: a multiple-electrode analysis of primate prefrontal cortex. J. Neurosci. 21, 3646–3655 (2001)
Aertsen, A. M. & Gerstein, G. L. Evaluation of neuronal connectivity: sensitivity of cross-correlation. Brain Res. 340, 341–354 (1985)
Dombeck, D. A., Graziano, M. S. & Tank, D. W. Functional clustering of neurons in motor cortex determined by cellular resolution imaging in awake behaving mice. J. Neurosci. 29, 13751–13760 (2009)
Shepherd, G. M. G., Stepanyants, A., Bureau, I., Chklovskii, D. B. & Svoboda, K. Geometric and functional organization of cortical circuits. Nature Neurosci. 8, 782–790 (2005)
Holmgren, C., Harkany, T., Svennenfors, B. & Zilberter, Y. Pyramidal cell communication within local networks in layer 2/3 of rat neocortex. J. Physiol. (Lond.) 551, 139–153 (2003)
Dobbins, E. G. & Feldman, J. L. Differential innervation of protruder and retractor muscles of the tongue in rat. J. Comp. Neurol. 357, 376–394 (1995)
Arenkiel, B. R. et al. In vivo light-induced activation of neural circuitry in transgenic mice expressing channelrhodopsin-2. Neuron 54, 205–218 (2007)
Hira, R. et al. Transcranial optogenetic stimulation for functional mapping of the motor cortex. J. Neurosci. Methods 179, 258–263 (2009)
Bodyak, N. & Slotnick, B. Performance of mice in an automated olfactometer: odor detection, discrimination and odor memory. Chem. Senses 24, 637–645 (1999)
Thévenaz, P., Ruttimann, U. E. & Unser, M. A pyramid approach to subpixel registration based on intensity. IEEE Trans. Image Process. 7, 27–41 (1998)
Svoboda, K., Denk, W., Kleinfeld, D. & Tank, D. W. In vivo dendritic calcium dynamics in neocortical pyramidal neurons. Nature 385, 161–165 (1997)
Clements, J. D. & Bekkers, J. M. Detection of spontaneous synaptic events with an optimally scaled template. Biophys. J. 73, 220–229 (1997)
Yaksi, E., Judkewitz, B. & Friedrich, R. W. Topological reorganization of odor representations in the olfactory bulb. PLoS Biol. 5, e178 (2007)
Acknowledgements
We thank D. Rinberg for help with experiments; F. Collman, D. Tank, C. Zuker, T. O’Connor and V. Iyer for help with analysis and imaging software; L. W. Enquist for pseudorabies vectors; W. Denk for help with mechanical design; D. Dombeck, M. Andermann, A. Kerlin and C. Reid for discussions about imaging awake mice; B. Shields, A. Hu and S. Michael for help with histology; A. Arnold for help with imaging; J. Osborne and S. Bassin for machining; L. Luo, Z. Mainen and D. Rinberg for comments on the manuscript; A. C. Gontang for illustration. Supported by Howard Hughes Medical Institute. T.K. is a Helen Hay Whitney Foundation postdoctoral fellow.
Author Contributions T.K. and K.S. conceived the project. T.K. developed and performed most of the experiments. D.H.O. helped to develop head-fixed behaviour. D.H. developed the glass-plug imaging window. Y.-X.Z. and D.H. performed optical stimulation mapping. T.K. and T.R.S. performed electrical stimulation mapping. T.K., B.M.H. and T.R.S. performed PRV tracing. T.K., T.R.S. and K.S. analysed data. M.G. provided a software module for image segmentation. T.K. and K.S. wrote the paper.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Figures
This file contains Supplementary Figures 1-17 with legends. (PDF 2614 kb)
Supplementary Movie 1
This movie shows normal voluntary licking of mice with muscimol injections in the somatosensory cortex. (MOV 658 kb)
Supplementary Movie 2
This movie shows normal voluntary licking of mice with muscimol injections in the anterior-medial cortex. (MOV 1446 kb)
Supplementary Movie 3
This movie shows defects in voluntary licking of mice with muscimol injections in alM. (MOV 1364 kb)
Supplementary Movie 4
This movie shows defects in voluntary licking of mice with muscimol injections in pmM. (MOV 2315 kb)
Rights and permissions
About this article
Cite this article
Komiyama, T., Sato, T., O’Connor, D. et al. Learning-related fine-scale specificity imaged in motor cortex circuits of behaving mice. Nature 464, 1182–1186 (2010). https://doi.org/10.1038/nature08897
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nature08897
This article is cited by
-
Arithmetic value representation for hierarchical behavior composition
Nature Neuroscience (2023)
-
Superior colliculus bidirectionally modulates choice activity in frontal cortex
Nature Communications (2023)
-
An adaptive behavioral control motif mediated by cortical axo-axonic inhibition
Nature Neuroscience (2023)
-
Robust odor identification in novel olfactory environments in mice
Nature Communications (2023)
-
Influence of Recent Trial History on Interval Timing
Neuroscience Bulletin (2023)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.