Abstract
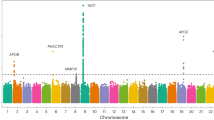
Sequence polymorphisms in a 58-kilobase (kb) interval on chromosome 9p21 confer a markedly increased risk of coronary artery disease (CAD), the leading cause of death worldwide1,2. The variants have a substantial effect on the epidemiology of CAD and other life-threatening vascular conditions because nearly one-quarter of Caucasians are homozygous for risk alleles. However, the risk interval is devoid of protein-coding genes and the mechanism linking the region to CAD risk has remained enigmatic. Here we show that deletion of the orthologous 70-kb non-coding interval on mouse chromosome 4 affects cardiac expression of neighbouring genes, as well as proliferation properties of vascular cells. Chr4Δ70kb/Δ70kb mice are viable, but show increased mortality both during development and as adults. Cardiac expression of two genes near the non-coding interval, Cdkn2a and Cdkn2b, is severely reduced in chr4Δ70kb/Δ70kb mice, indicating that distant-acting gene regulatory functions are located in the non-coding CAD risk interval. Allele-specific expression of Cdkn2b transcripts in heterozygous mice showed that the deletion affects expression through a cis-acting mechanism. Primary cultures of chr4Δ70kb/Δ70kb aortic smooth muscle cells exhibited excessive proliferation and diminished senescence, a cellular phenotype consistent with accelerated CAD pathogenesis. Taken together, our results provide direct evidence that the CAD risk interval has a pivotal role in regulation of cardiac Cdkn2a/b expression, and suggest that this region affects CAD progression by altering the dynamics of vascular cell proliferation.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Helgadottir, A. et al. A common variant on chromosome 9p21 affects the risk of myocardial infarction. Science 316, 1491–1493 (2007)
McPherson, R. et al. A common allele on chromosome 9 associated with coronary heart disease. Science 316, 1488–1491 (2007)
Lloyd-Jones, D. et al. Heart disease and stroke statistics—2009 update. A report from the American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Circulation 119, e21–e181 (2009)
Topol, E. J., Smith, J., Plow, E. F. & Wang, Q. K. Genetic susceptibility to myocardial infarction and coronary artery disease. Hum. Mol. Genet. 15, R117–R123 (2006)
Schunkert, H. et al. Repeated replication and a prospective meta-analysis of the association between chromosome 9p21.3 and coronary artery disease. Circulation 117, 1675–1684 (2008)
Hinohara, K. et al. Replication of the association between a chromosome 9p21 polymorphism and coronary artery disease in Japanese and Korean populations. J. Hum. Genet. 53, 357–359 (2008)
Lemmens, R. et al. Variant on 9p21 strongly associates with coronary heart disease, but lacks association with common stroke. Eur. J Hum. Genet. 17, 1287–1293 (2009)
Shen, G. Q. et al. Association between four SNPs on chromosome 9p21 and myocardial infarction is replicated in an Italian population. J. Hum. Genet. 53, 144–150 (2008)
Zhou, L. et al. Associations between single nucleotide polymorphisms on chromosome 9p21 and risk of coronary heart disease in Chinese Han population. Arterioscler. Thromb. Vasc. Biol. 28, 2085–2089 (2008)
Helgadottir, A. et al. The same sequence variant on 9p21 associates with myocardial infarction, abdominal aortic aneurysm and intracranial aneurysm. Nature Genet. 40, 217–224 (2008)
Guttman, M. et al. Chromatin signature reveals over a thousand highly conserved large non-coding RNAs in mammals. Nature 458, 223–227 (2009)
Pasmant, E. et al. Characterization of a germ-line deletion, including the entire INK4/ARF locus, in a melanoma-neural system tumor family: identification of ANRIL, an antisense non-coding RNA whose expression coclusters with ARF. Cancer Res. 67, 3963–3969 (2007)
Jarinova, O. et al. Functional analysis of the chromosome 9p21.3 coronary artery disease risk locus. Arterioscler. Thromb. Vasc. Biol. 29, 1671–1677 (2009)
Liu, Y. et al. INK4/ARF transcript expression is associated with chromosome 9p21 variants linked to atherosclerosis. PLoS One 4, e5027 (2009)
Karolchik, D. et al. The UCSC Genome Browser Database: 2008 update. Nucleic Acids Res. 36, D773–D779 (2008)
Serrano, M. et al. Role of the INK4a locus in tumor suppression and cell mortality. Cell 85, 27–37 (1996)
Kamijo, T. et al. Tumor suppression at the mouse INK4a locus mediated by the alternative reading frame product p19ARF. Cell 91, 649–659 (1997)
Sharpless, N. E., Ramsey, M. R., Balasubramanian, P., Castrillon, D. H. & DePinho, R. A. The differential impact of p16INK4a or p19ARF deficiency on cell growth and tumorigenesis. Oncogene 23, 379–385 (2004)
Latres, E. et al. Limited overlapping roles of P15INK4b and P18INK4c cell cycle inhibitors in proliferation and tumorigenesis. EMBO J. 19, 3496–3506 (2000)
Besson, A., Assoian, R. K. & Roberts, J. M. Regulation of the cytoskeleton: an oncogenic function for CDK inhibitors? Nature Rev. Cancer 4, 948–955 (2004)
Paigen, B., Morrow, A., Brandon, C., Mitchell, D. & Holmes, P. Variation in susceptibility to atherosclerosis among inbred strains of mice. Atherosclerosis 57, 65–73 (1985)
Plump, A. S. et al. Severe hypercholesterolemia and atherosclerosis in apolipoprotein E-deficient mice created by homologous recombination in ES cells. Cell 71, 343–353 (1992)
Zhang, S. H., Reddick, R. L., Piedrahita, J. A. & Maeda, N. Spontaneous hypercholesterolemia and arterial lesions in mice lacking apolipoprotein E. Science 258, 468–471 (1992)
Broadbent, H. M. et al. Susceptibility to coronary artery disease and diabetes is encoded by distinct, tightly linked SNPs in the ANRIL locus on chromosome 9p. Hum. Mol. Genet. 17, 806–814 (2008)
Gizard, F. et al. PPARα inhibits vascular smooth muscle cell proliferation underlying intimal hyperplasia by inducing the tumor suppressor p16INK4a. J. Clin. Invest. 115, 3228–3238 (2005)
Diéz-Juan, A. & Andres, V. The growth suppressor p27Kip1 protects against diet-induced atherosclerosis. FASEB J. 15, 1989–1995 (2001)
González, P. et al. A single-nucleotide polymorphism in the human p27kip1 gene (-838C>A) affects basal promoter activity and the risk of myocardial infarction. BMC Biol. 2, 5 (2004)
Rodríguez, I. et al. Role of the CDKN1A/p21, CDKN1C/p57, and CDKN2A/p16 genes in the risk of atherosclerosis and myocardial infarction. Cell Cycle 6, 620–625 (2007)
Yoshida, T., Kaestner, K. H. & Owens, G. K. Conditional deletion of Kruppel-like factor 4 delays downregulation of smooth muscle cell differentiation markers but accelerates neointimal formation following vascular injury. Circ. Res. 102, 1548–1557 (2008)
Dimri, G. P. et al. A biomarker that identifies senescent human cells in culture and in aging skin in vivo.. Proc. Natl Acad. Sci. USA 92, 9363–9367 (1995)
Ueda, Y. et al. Relationship between expression levels and atherogenesis in scavenger receptor class B, type I transgenics. J. Biol. Chem. 275, 20368–20373 (2000)
Acknowledgements
We thank G. Owens for critical suggestions and discussion, T. Ley for providing vector pTURBO-Cre, and D. Lee for help with plasma lipid analysis. L.A.P., E.M.R. and J.C.C. were supported by the National Heart, Lung, and Blood Institute, D.M. and C.A. by EMBO long-term fellowships and L.A.P. by the National Human Genome Research Institute. Research was conducted at the E.O. Lawrence Berkeley National Laboratory and performed under Department of Energy Contract DE-AC02-05CH11231, University of California. Plasma lipid analysis at the University of Cincinnati Mouse Metabolic Phenotyping Center was supported by MMPC DK59630. All animal work was reviewed and approved by the LBNL Animal Welfare and Research Committee.
Author Contributions A.V. and L.A.P. wrote the manuscript. All authors contributed to data collection and analysis and provided comments on the manuscript.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Information
This file contains Supplementary Text, Supplementary Figures 1- 9 with Legends, Supplementary Tables 1- 4 and Supplementary References. (PDF 1484 kb)
Rights and permissions
About this article
Cite this article
Visel, A., Zhu, Y., May, D. et al. Targeted deletion of the 9p21 non-coding coronary artery disease risk interval in mice. Nature 464, 409–412 (2010). https://doi.org/10.1038/nature08801
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nature08801
This article is cited by
-
Killing the two deadly birds of atherosclerosis and cancer with one stone
Nature Cardiovascular Research (2022)
-
The effect of polymorphisms (174G> C and 572C> G) on the Interleukin-6 gene in coronary artery disease: a systematic review and meta-analysis
Genes and Environment (2021)
-
Multivariate genomic scan implicates novel loci and haem metabolism in human ageing
Nature Communications (2020)
-
The Pivotal Role of Senescence in Cell Death and Aging: Where Do We Stand?
Current Molecular Biology Reports (2020)
-
Nucleic Acid–Based Therapies for Atherosclerosis
Current Atherosclerosis Reports (2020)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.