Abstract
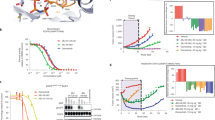
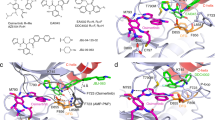
The clinical efficacy of epidermal growth factor receptor (EGFR) kinase inhibitors in EGFR-mutant non-small-cell lung cancer (NSCLC) is limited by the development of drug-resistance mutations, including the gatekeeper T790M mutation1,2,3. Strategies targeting EGFR T790M with irreversible inhibitors have had limited success and are associated with toxicity due to concurrent inhibition of wild-type EGFR4,5. All current EGFR inhibitors possess a structurally related quinazoline-based core scaffold and were identified as ATP-competitive inhibitors of wild-type EGFR. Here we identify a covalent pyrimidine EGFR inhibitor by screening an irreversible kinase inhibitor library specifically against EGFR T790M. These agents are 30- to 100-fold more potent against EGFR T790M, and up to 100-fold less potent against wild-type EGFR, than quinazoline-based EGFR inhibitors in vitro. They are also effective in murine models of lung cancer driven by EGFR T790M. Co-crystallization studies reveal a structural basis for the increased potency and mutant selectivity of these agents. These mutant-selective irreversible EGFR kinase inhibitors may be clinically more effective and better tolerated than quinazoline-based inhibitors. Our findings demonstrate that functional pharmacological screens against clinically important mutant kinases represent a powerful strategy to identify new classes of mutant-selective kinase inhibitors.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Mok, T. S. et al. Gefitinib or carboplatin-paclitaxel in pulmonary adenocarcinoma. N. Engl. J. Med. 361, 947–957 (2009)
Pao, W. et al. Acquired resistance of lung adenocarcinomas to gefitinib or erlotinib is associated with a second mutation in the EGFR kinase domain. PLoS Med. 2, 1–11 (2005)
Kobayashi, S. et al. EGFR mutation and resistance of non-small-cell lung cancer to gefitinib. N. Engl. J. Med. 352, 786–792 (2005)
Li, D. et al. Bronchial and peripheral murine lung carcinomas induced by T790M–L858R mutant EGFR respond to HKI-272 and rapamycin combination therapy. Cancer Cell 12, 81–93 (2007)
Besse, B. et al. Neratinib (HKI-272), an irreversible pan-ErbB receptor tyrosine kinase inhibitor: preliminary results of a phase 2 trial in patients with advanced non-small cell lung cancer. Eur. J. Cancer Suppl. 6, 64, abstr. 203 (2008)
Rosell, R. et al. Screening for epidermal growth factor receptor mutations in lung cancer. N. Engl. J. Med. 361, 958–967 (2009)
Yun, C. H. et al. Structures of lung cancer-derived EGFR mutants and inhibitor complexes: mechanism of activation and insights into differential inhibitor sensitivity. Cancer Cell 11, 217–227 (2007)
Carey, K. D. et al. Kinetic analysis of epidermal growth factor receptor somatic mutant proteins shows increased sensitivity to the epidermal growth factor receptor tyrosine kinase inhibitor, erlotinib. Cancer Res. 66, 8163–8171 (2006)
Yun, C. H. et al. The T790M mutation in EGFR kinase causes drug resistance by increasing the affinity for ATP. Proc. Natl Acad. Sci. USA 105, 2070–2075 (2008)
Engelman, J. A. et al. PF00299804, an irreversible pan-ERBB inhibitor, is effective in lung cancer models with EGFR and ERBB2 mutations that are resistant to gefitinib. Cancer Res. 67, 11924–11932 (2007)
Li, D. et al. BIBW2992, an irreversible EGFR/HER2 inhibitor highly effective in preclinical lung cancer models. Oncogene 27, 4702–4711 (2008)
Yuza, Y. et al. Allele-dependent variation in the relative cellular potency of distinct EGFR inhibitors. Cancer Biol. Ther. 6, 661–667 (2007)
Godin-Heymann, N. et al. The T790M ‘gatekeeper’ mutation in EGFR mediates resistance to low concentrations of an irreversible EGFR inhibitor. Mol. Cancer Ther. 7, 874–879 (2008)
Janne, P. A. et al. Preliminary activity and safety results from a phase I clinical trial of PF-00299804, an irreversible pan-HER inhibitor, in patients (pts) with NSCLC. J. Clin. Oncol. 26 (Suppl.), abstr 8027 (2008)
Janne, P. A. et al. Multicenter, randomized, phase II trial of CI-1033, an irreversible pan-ERBB inhibitor, for previously treated advanced non small-cell lung cancer. J. Clin. Oncol. 25, 3936–3944 (2007)
Hur, W. et al. Clinical stage EGFR inhibitors irreversibly alkylate Bmx kinase. Bioorg. Med. Chem. Lett. 18, 5916–5919 (2008)
Yonesaka, K. et al. Autocrine production of amphiregulin predicts sensitivity to both gefitinib and cetuximab in EGFR wild-type cancers. Clin. Cancer Res. 14, 6963–6973 (2008)
Breault, G. A. et al. Cyclin-dependent kinase 4 inhibitors as a treatment for cancer. Part 2: identification and optimisation of substituted 2,4-bis anilino pyrimidines. Bioorg. Med. Chem. Lett. 13, 2961–2966 (2003)
Alam, M. et al. Synthesis and SAR of aminopyrimidines as novel c-Jun N-terminal kinase (JNK) inhibitors. Bioorg. Med. Chem. Lett. 17, 3463–3467 (2007)
Lietha, D. & Eck, M. J. Crystal structures of the FAK kinase in complex with TAE226 and related bis-anilino pyrimidine inhibitors reveal a helical DFG conformation. PLoS One 3, e3800 (2008)
Ogino, A. et al. Emergence of epidermal growth factor receptor T790M mutation during chronic exposure to gefitinib in a non small cell lung cancer cell line. Cancer Res. 67, 7807–7814 (2007)
Engelman, J. A. et al. Allelic dilution obscures detection of a biologically significant resistance mutation in EGFR-amplified lung cancer. J. Clin. Invest. 116, 2695–2706 (2006)
Tsai, J. et al. Discovery of a selective inhibitor of oncogenic B-Raf kinase with potent antimelanoma activity. Proc. Natl Acad. Sci. USA 105, 3041–3046 (2008)
O’Hare, T. et al. SGX393 inhibits the CML mutant Bcr-AblT315I and preempts in vitro resistance when combined with nilotinib or dasatinib. Proc. Natl Acad. Sci. USA 105, 5507–5512 (2008)
Gontarewicz, A. et al. PHA-680626 exhibits anti-proliferative and pro-apoptotic activity on imatinib-resistant chronic myeloid leukemia cell lines and primary CD34+ cells by inhibition of both Bcr-Abl tyrosine kinase and Aurora kinases. Leuk. Res. 32, 1857–1865 (2008)
Engelman, J. A. et al. MET amplification leads to gefitinib resistance in lung cancer by activating ERBB3 signaling. Science 316, 1039–1043 (2007)
Ji, H. et al. The impact of human EGFR kinase domain mutations on lung tumorigenesis and in vivo sensitivity to EGFR-targeted therapies. Cancer Cell 9, 485–495 (2006)
Bradeen, H. A. et al. Comparison of imatinib mesylate, dasatinib (BMS-354825), and nilotinib (AMN107) in an N-ethyl-N-nitrosourea (ENU)-based mutagenesis screen: high efficacy of drug combinations. Blood 108, 2332–2338 (2006)
Acknowledgements
This study is supported by grants from the National Institutes of Health RO1CA11446 (P.A.J.), R01CA135257 (P.A.J.), R01CA080942 (M.J.E.), R01CA130876-02 (N.S.G.), R01CA116020 (M.J.E.), R01AG2400401 (K.-K.W.), R01 CA122794 (K.-K.W.), R01GM070590 (J.R.E.), National Cancer Institute Lung SPORE P50CA090578 (P.A.J. and K.-K.W.), the Cecily and Robert Harris Foundation (K.-K.W.), Uniting Against Lung Cancer (K.-K.W.), the Flight Attendant Medical Research Institute (K.-K.W.)., the Hazel and Samuel Bellin Research Fund (P.A.J.) and the Damon Runyon Foundation Cancer Innovation Award (N.S.G.). This work is contribution 948 from the Barnett Institute. The authors thank Sai Advantium Pharma for performing the pharmacokinetic studies.
Author Contributions W.Z., D.E., Li.C., C.-H.Y., D.L., M.C. A.B.C. designed experiments, conducted studies and analysed data. R.E.I. and J.R.E. performed and analysed the mass spectrometry studies. Lu.C. and R.P. performed the histological and immunohistochemistry analyses. M.J.E., K.-K.W., N.S.G. and P.A.J. designed the experiments, analysed data and wrote the manuscript. The Wong, Eck, Gray and Jänne laboratories contributed equally to this work.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Competing interests
P.A.J. receives royalties as a co-inventor on a patent awarded for the discovery of EGFR mutations, licensed to Genzyme Genetics, which was not involved in this study. M.J.E. is a consultant for and receives research support from Novartis Institutes for Biomedical Research. N.S.G., M.J.E. and P.A.J. are co-inventors on a provisional patent application covering the inhibitors described in the manuscript which have been licensed to Gatekeeper Pharmaceuticals of which N.S.G., K.-K.W. and P.A.J. are co-founders.
Supplementary information
Supplementary Information
This file contains Supplementary Methods, Supplementary Tables S1- S10, Supplementary Figures S1-S11 with Legends and Supplementary References. (PDF 1228 kb)
Rights and permissions
About this article
Cite this article
Zhou, W., Ercan, D., Chen, L. et al. Novel mutant-selective EGFR kinase inhibitors against EGFR T790M. Nature 462, 1070–1074 (2009). https://doi.org/10.1038/nature08622
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1038/nature08622
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.