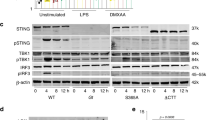
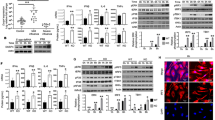
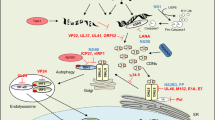
Abstract
The innate immune system is critical for the early detection of invading pathogens and for initiating cellular host defence countermeasures, which include the production of type I interferon (IFN)1,2,3. However, little is known about how the innate immune system is galvanized to respond to DNA-based microbes. Here we show that STING (stimulator of interferon genes) is critical for the induction of IFN by non-CpG intracellular DNA species produced by various DNA pathogens after infection4. Murine embryonic fibroblasts, as well as antigen presenting cells such as macrophages and dendritic cells (exposed to intracellular B-form DNA, the DNA virus herpes simplex virus 1 (HSV-1) or bacteria Listeria monocytogenes), were found to require STING to initiate effective IFN production. Accordingly, Sting-knockout mice were susceptible to lethal infection after exposure to HSV-1. The importance of STING in facilitating DNA-mediated innate immune responses was further evident because cytotoxic T-cell responses induced by plasmid DNA vaccination were reduced in Sting-deficient animals. In the presence of intracellular DNA, STING relocalized with TANK-binding kinase 1 (TBK1) from the endoplasmic reticulum to perinuclear vesicles containing the exocyst component Sec5 (also known as EXOC2). Collectively, our studies indicate that STING is essential for host defence against DNA pathogens such as HSV-1 and facilitates the adjuvant activity of DNA-based vaccines.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Palm, N. W. & Medzhitov, R. Pattern recognition receptors and control of adaptive immunity. Immunol. Rev. 227, 221–233 (2009)
Takeuchi, O. & Akira, S. Innate immunity to virus infection. Immunol. Rev. 227, 75–86 (2009)
Beutler, B. A. TLRs and innate immunity. Blood 113, 1399–1407 (2009)
Ishikawa, H. & Barber, G. N. STING is an endoplasmic reticulum adaptor that facilitates innate immune signalling. Nature 455, 674–678 (2008)
Kato, H. et al. Differential roles of MDA5 and RIG-I helicases in the recognition of RNA viruses. Nature 441, 101–105 (2006)
Yoneyama, M. et al. The RNA helicase RIG-I has an essential function in double-stranded RNA-induced innate antiviral responses. Nature Immunol. 5, 730–737 (2004)
Kawai, T. et al. IPS-1, an adaptor triggering RIG-I- and Mda5-mediated type I interferon induction. Nature Immunol. 6, 981–988 (2005)
Seth, R. B., Sun, L., Ea, C. K. & Chen, Z. J. Identification and characterization of MAVS, a mitochondrial antiviral signaling protein that activates NF-κB and IRF 3. Cell 122, 669–682 (2005)
Meylan, E. et al. Cardif is an adaptor protein in the RIG-I antiviral pathway and is targeted by hepatitis C virus. Nature 437, 1167–1172 (2005)
Xu, L. G. et al. VISA is an adapter protein required for virus-triggered IFN-β signaling. Mol. Cell 19, 727–740 (2005)
Bauer, S., Pigisch, S., Hangel, D., Kaufmann, A. & Hamm, S. Recognition of nucleic acid and nucleic acid analogs by Toll-like receptors 7, 8 and 9. Immunobiology 213, 315–328 (2008)
Ishii, K. J. et al. TANK-binding kinase-1 delineates innate and adaptive immune responses to DNA vaccines. Nature 451, 725–729 (2008)
Takaoka, A. et al. DAI (DLM-1/ZBP1) is a cytosolic DNA sensor and an activator of innate immune response. Nature 448, 501–505 (2007)
Muruve, D. A. et al. The inflammasome recognizes cytosolic microbial and host DNA and triggers an innate immune response. Nature 452, 103–107 (2008)
Roberts, T. L. et al. HIN-200 proteins regulate caspase activation in response to foreign cytoplasmic DNA. Science 323, 1057–1060 (2009)
Hornung, V. et al. AIM2 recognizes cytosolic dsDNA and forms a caspase-1-activating inflammasome with ASC. Nature 458, 514–518 (2009)
Fernandes-Alnemri, T., Yu, J. W., Datta, P., Wu, J. & Alnemri, E. S. AIM2 activates the inflammasome and cell death in response to cytoplasmic DNA. Nature 458, 509–513 (2009)
Bürckstüümmer, T. et al. An orthogonal proteomic-genomic screen identifies AIM2 as a cytoplasmic DNA sensor for the inflammasome. Nature Immunol. 10, 266–272 (2009)
Jin, L. et al. MPYS, a novel membrane tetraspanner, is associated with major histocompatibility complex class II and mediates transduction of apoptotic signals. Mol. Cell. Biol. 28, 5014–5026 (2008)
Zhong, B. et al. The adaptor protein MITA links virus-sensing receptors to IRF3 transcription factor activation. Immunity 29, 538–550 (2008)
Ablasser, A. et al. RIG-I-dependent sensing of poly(dA:dT) through the induction of an RNA polymerase III-transcribed RNA intermediate. Nature Immunol. 10.1038/ni.1779 (16 July 2009)
Chiu, Y. H., Macmillan, J. B. & Chen, Z. J. RNA polymerase III detects cytosolic dna and induces type I interferons through the RIG-I pathway. Cell 138, 576–591 (2009)
Saito, T., Owen, D. M., Jiang, F., Marcotrigiano, J. & Gale, M. Innate immunity induced by composition-dependent RIG-I recognition of hepatitis C virus RNA. Nature 454, 523–527 (2008)
Muñoz-Jordan, J. L. et al. Inhibition of α/β interferon signaling by the NS4B protein of flaviviruses. J. Virol. 79, 8004–8013 (2005)
Spies, B. et al. Vaccination with plasmid DNA activates dendritic cells via Toll-like receptor 9 (TLR9) but functions in TLR9-deficient mice. J. Immunol. 171, 5908–5912 (2003)
Ménétret, J. F. et al. Single copies of Sec61 and TRAP associate with a nontranslating mammalian ribosome. Structure 16, 1126–1137 (2008)
Hayashi, T., Rizzuto, R., Hajnoczky, G. & Su, T. P. MAM: more than just a housekeeper. Trends Cell Biol. 19, 81–88 (2009)
Chien, Y. et al. RalB GTPase-mediated activation of the IκB family kinase TBK1 couples innate immune signaling to tumor cell survival. Cell 127, 157–170 (2006)
Spiczka, K. S. & Yeaman, C. Ral-regulated interaction between Sec5 and paxillin targets Exocyst to focal complexes during cell migration. J. Cell Sci. 121, 2880–2891 (2008)
Mavinakere, M. S., Williamson, C. D., Goldmacher, V. S. & Colberg-Poley, A. M. Processing of human cytomegalovirus UL37 mutant glycoproteins in the endoplasmic reticulum lumen prior to mitochondrial importation. J. Virol. 80, 6771–6783 (2006)
Acknowledgements
We thank J. Yewdell for VV-OVA, B. Jacobs for VVΔE3L, K. Frueh for HCMV, M. Kobayashi for baculovirus, H. Horiuchi for the Sec5 antibody, Y. C. Weh for Tbk1-knockout MEFs, and S. Nagata, T. Maniatis, J. Hiscott and N. Reich for plasmid constructs. This work was supported by NIH grant AI079336.
Author Contributions H.I. and G.N.B. designed the research and analysed the data. H.I. performed most experiments. Z.M. performed experiments related to YFV NS4B, carried out exocyst RNAi studies and helped with experiments. G.N.B. wrote the paper.
Author information
Authors and Affiliations
Corresponding author
Supplementary information
Supplementary Figures
This file contains Supplementary Figures 1-8 with Legends. (PDF 10042 kb)
Rights and permissions
About this article
Cite this article
Ishikawa, H., Ma, Z. & Barber, G. STING regulates intracellular DNA-mediated, type I interferon-dependent innate immunity. Nature 461, 788–792 (2009). https://doi.org/10.1038/nature08476
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nature08476
This article is cited by
-
Interferon-induced transmembrane protein-1 competitively blocks Ephrin receptor A2-mediated Epstein–Barr virus entry into epithelial cells
Nature Microbiology (2024)
-
Activating STING/TBK1 suppresses tumor growth via degrading HPV16/18 E7 oncoproteins in cervical cancer
Cell Death & Differentiation (2024)
-
Single-molecule localization microscopy reveals STING clustering at the trans-Golgi network through palmitoylation-dependent accumulation of cholesterol
Nature Communications (2024)
-
The tumor cell-intrinsic cGAS–STING pathway is associated with the high density of CD8+ T cells after chemotherapy in esophageal squamous cell carcinoma
Esophagus (2024)
-
SAM68 directs STING signaling to apoptosis in macrophages
Communications Biology (2024)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.