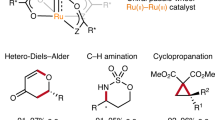
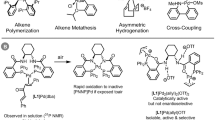
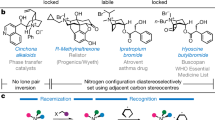
Abstract
Discovery of efficient catalysts is one of the most compelling objectives of modern chemistry. Chiral catalysts are in particularly high demand, as they facilitate synthesis of enantiomerically enriched small molecules that are critical to developments in medicine, biology and materials science1. Especially noteworthy are catalysts that promote—with otherwise inaccessible efficiency and selectivity levels—reactions demonstrated to be of great utility in chemical synthesis. Here we report a class of chiral catalysts that initiate alkene metathesis1 with very high efficiency and enantioselectivity. Such attributes arise from structural fluxionality of the chiral catalysts and the central role that enhanced electronic factors have in the catalytic cycle. The new catalysts have a stereogenic metal centre and carry only monodentate ligands; the molybdenum-based complexes are prepared stereoselectively by a ligand exchange process involving an enantiomerically pure aryloxide, a class of ligands scarcely used in enantioselective catalysis2,3. We demonstrate the application of the new catalysts in an enantioselective synthesis of the Aspidosperma alkaloid, quebrachamine, through an alkene metathesis reaction that cannot be promoted by any of the previously reported chiral catalysts.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Hoveyda, A. H. & Zhugralin, A. R. The remarkable metal-catalysed olefin metathesis reaction. Nature 450, 243–251 (2007)
Hashimoto, S., Komeshima, N. & Koga, K. Asymmetric Diels–Alder reaction catalysed by chiral alkoxyaluminum dichloride. J. Chem. Soc. Chem. Commun. 437–438 (1979)
Tayama, E., Saito, A., Ooi, T. & Maruoka, K. Activation of ether functionality of allyl vinyl ethers by chiral bis(organoaluminum) Lewis acids: application to asymmetric Claisen rearrangement. Tetrahedron 58, 8307–8312 (2002)
Solans-Monfort, X., Clot, E., Copéret, C. & Eisenstein, O. d0-Re-based olefin metathesis catalysts, Re(≡CR)( = CHR)(X)(Y): the key role of X and Y ligands for efficient active sites. J. Am. Chem. Soc. 127, 14015–14025 (2005)
Poater, A., Solans-Monfort, X., Clot, E., Copéret, C. & Eisenstein, O. Understanding d0-olefin metathesis catalysts: Which metal, which ligands? J. Am. Chem. Soc. 129, 8207–8216 (2007)
Brunner, H. & Prommesberger, M. Asymmetric catalysis. Part 127: Enantioselective desymmetrization of 2-n-butyl-4,7-dihydro-1,3-dioxepin with (η6-arene)ruthenium(II) half-sandwich complexes. Tetrahedr. Asymm. 9, 3231–3239 (1999)
Faller, J. W., Grimmond, B. J. & D’Alliessi, D. G. An application of electronic asymmetry to highly enantioselective catalytic Diels-Alder reactions. J. Am. Chem. Soc. 123, 2525–2529 (2001)
Noyori, R. Asymmetric catalysis: science and opportunities (Nobel lecture). Angew. Chem. Int. Ed. 41, 2008–2022 (2002)
Fontecave, M., Hamelin, O. & Ménage, S. Chiral-at-metal complexes as asymmetric catalysts. Top. Organomet. Chem. 15, 271–278 (2005)
Brunner, H. Optically active organometallic compounds of transition elements with chiral metal atoms. Angew. Chem. Int. Ed. 38, 1194–1208 (1999)
Brunner, H. & Fisch, K. Catalytic hydrosilylation or hydrogenation at one coordination site of [Cp’Fe(CO)(X)] fragments. Angew. Chem. Int. Ed. 29, 1131–1132 (1990)
Schrock, R. R. & Hoveyda, A. H. Molybdenum and tungsten imido alkylidene complexes as efficient olefin-metathesis catalysts. Angew. Chem. Int. Ed. 42, 4592–4633 (2003)
Grubbs, R. H. Handbook of Metathesis (Wiley-VCH, 2003)
Kozmin, S. A., Iwama, T., Huang, Y. & Rawal, V. H. An efficient approach to Aspidosperma alkaloids via [4+2] cycloadditions of aminosiloxydienes: Stereocontrolled total syntheses of (±)-tabersonine. Gram-scale catalytic asymmetric syntheses of (+)-tabersonine and (+)-16-methoxytabersonine. Asymmetric syntheses of (+)-aspidospermidine and (–)-quebrachamine. J. Am. Chem. Soc. 124, 4628–4641 (2002)
Deutsch, H. F., Evenson, M. A., Drescher, P., Sparwasser, C. & Madsen, P. Isolation and biological activity of aspidospermine and quebrachamine from an Aspidosperma tree source. J. Pharm. Biomed. Anal. 12, 1283–1287 (1994)
Schrock, R. R. et al. Synthesis of molybdenum imido alkylidene complexes and some reactions involving acyclic olefins. J. Am. Chem. Soc. 112, 3875–3886 (1990)
Garber, S. B., Kingsbury, J. S., Gray, B. L. & Hoveyda, A. H. Efficient and recyclable monomeric and dendritic Ru-based metathesis catalysts. J. Am. Chem. Soc. 122, 8168–8179 (2000)
Stewart, I. C., Douglas, C. J. & Grubbs, R. H. Increased efficiency in cross-metathesis reactions of sterically hindered olefins. Org. Lett. 10, 441–444 (2008)
Van Veldhuizen, J. J., Campbell, J. E., Giudici, R. E. & Hoveyda, A. H. A readily available chiral Ag-based N-heterocyclic carbene complex for use in efficient and highly enantioselective Ru-catalyzed olefin metathesis and Cu-catalyzed allylic alkylation reactions. J. Am. Chem. Soc. 127, 6877–6882 (2005)
Funk, T. W., Berlin, J. M. & Grubbs, R. H. Highly active chiral ruthenium catalysts for asymmetric ring-closing olefin metathesis. J. Am. Chem. Soc. 128, 1840–1846 (2006)
Alexander, J. B., La, D. S., Cefalo, D. R., Hoveyda, A. H. & Schrock, R. R. Catalytic enantioselective ring-closing metathesis by a chiral biphen-Mo complex. J. Am. Chem. Soc. 120, 4041–4042 (1998)
Tsang, W. C. P. et al. Alkylidene and metalacyclic complexes of tungsten that contain a chiral biphenoxide ligand. Synthesis, asymmetric ring-closing metathesis, and mechanistic investigations. J. Am. Chem. Soc. 125, 2652–2666 (2003)
Singh, R., Schrock, R. R., Müller, P. & Hoveyda, A. H. Synthesis of monoalkoxide monopyrrolyl complexes of the type Mo(NR)(CHR')(OR'')(pyrrolyl). Enyne metathesis with high oxidation state catalysts. J. Am. Chem. Soc. 129, 12654–12655 (2007)
Hock, A. S., Schrock, R. R. & Hoveyda, A. H. Dipyrrolyl precursors to bisalkoxide molybdenum olefin metathesis catalysts. J. Am. Chem. Soc. 128, 16373–16375 (2006)
Dolman, S. J., Sattely, E. S., Hoveyda, A. H. & Schrock, R. R. Efficient catalytic enantioselective synthesis of unsaturated amines: preparation of small- and medium-ring cyclic amines through Mo–catalyzed asymmetric ring-closing metathesis in the absence of solvent. J. Am. Chem. Soc. 124, 6991–6997 (2002)
Sattely, E. S., Cortez, G. A., Moebius, D. C., Schrock, R. R. & Hoveyda, A. H. Enantioselective synthesis of cyclic amides and amines through Mo–catalyzed asymmetric ring-closing metathesis. J. Am. Chem. Soc. 127, 8526–8533 (2005)
Kiely, A. F., Jernelius, J. A., Schrock, R. R. & Hoveyda, A. H. Enantioselective synthesis of medium-ring heterocycles, tertiary ethers, and tertiary alcohols by Mo–catalyzed ring-closing metathesis. J. Am. Chem. Soc. 124, 2868–2869 (2002)
Van Veldhuizen, J. J., Gillingham, D. G., Garber, S. B., Kataoka, O. & Hoveyda, A. H. Chiral Ru-based complexes for asymmetric olefin metathesis: Enhancement of catalyst activity through steric and electronic modifications. J. Am. Chem. Soc. 125, 12502–12508 (2003)
Fournier, P.-A. & Collins, S. K. A highly active chiral ruthenium-based catalyst for enantioselective olefin metathesis. Organometallics 26, 2945–2949 (2007)
Acknowledgements
This research was supported by the US National Institutes of Health, Institute of General Medical Sciences (grant GM-59426 to A.H.H. and R.R.S.). We are grateful to B. C. Bailey and K. Wampler for assistance in obtaining the X-ray structure of the major diastereomer of molybdenum complex 13b. We thank A. R. Zhugralin for numerous discussions regarding the mechanistic aspects of these investigations and R. Singh for experimental suggestions. Mass spectrometry facilities at Boston College are supported by the US National Science Foundation (grant DBI-0619576).
Author Contributions S. J. Malcolmson and S. J. Meek were involved in the discovery and development of the new catalysts. E.S.S. designed and developed the synthesis route to racemic quebrachamine. A.H.H. and R.R.S. designed and directed the research program. A.H.H. wrote the manuscript.
Author information
Authors and Affiliations
Corresponding author
Additional information
X-ray crystallographic data have been deposited at the Cambridge Crystallographic Data Centre, UK; CCDC 703841 contains the supplementary crystallographic data for this paper. These data can be obtained free of charge from The Cambridge Crystallographic Data Centre (www.ccdc.cam.ac.uk/data_request/cif).
Supplementary information
Supplementary Information
This file contains Supplementary Notes with Supplementary Tables 1-7 and Supporting Information. (PDF 4218 kb)
Rights and permissions
About this article
Cite this article
Malcolmson, S., Meek, S., Sattely, E. et al. Highly efficient molybdenum-based catalysts for enantioselective alkene metathesis. Nature 456, 933–937 (2008). https://doi.org/10.1038/nature07594
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nature07594
This article is cited by
-
Olefin metathesis of fatty acids and vegetable oils
Journal of Chemical Sciences (2019)
-
Ring-opening polymerization of l-lactide catalyzed by a novel molybdenum-based catalytic system
Iranian Polymer Journal (2018)
-
Molybdenum chloride catalysts for Z-selective olefin metathesis reactions
Nature (2017)
-
Bisguanidinium dinuclear oxodiperoxomolybdosulfate ion pair-catalyzed enantioselective sulfoxidation
Nature Communications (2016)
-
Catalytic enantioselective synthesis of quaternary carbon stereocentres
Nature (2014)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.