Abstract
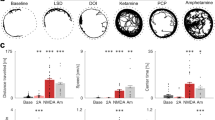
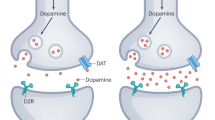
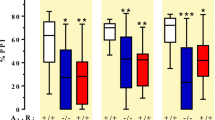
The psychosis associated with schizophrenia is characterized by alterations in sensory processing and perception1,2. Some antipsychotic drugs were identified by their high affinity for serotonin 5-HT2A receptors (2AR)3,4. Drugs that interact with metabotropic glutamate receptors (mGluR) also have potential for the treatment of schizophrenia5,6,7. The effects of hallucinogenic drugs, such as psilocybin and lysergic acid diethylamide, require the 2AR8,9,10 and resemble some of the core symptoms of schizophrenia10,11,12. Here we show that the mGluR2 interacts through specific transmembrane helix domains with the 2AR, a member of an unrelated G-protein-coupled receptor family, to form functional complexes in brain cortex. The 2AR–mGluR2 complex triggers unique cellular responses when targeted by hallucinogenic drugs, and activation of mGluR2 abolishes hallucinogen-specific signalling and behavioural responses. In post-mortem human brain from untreated schizophrenic subjects, the 2AR is upregulated and the mGluR2 is downregulated, a pattern that could predispose to psychosis. These regulatory changes indicate that the 2AR–mGluR2 complex may be involved in the altered cortical processes of schizophrenia, and this complex is therefore a promising new target for the treatment of psychosis.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Freedman, R. Schizophrenia. N. Engl. J. Med. 349, 1738–1749 (2003)
Sawa, A. & Snyder, S. H. Schizophrenia: diverse approaches to a complex disease. Science 296, 692–695 (2002)
Lieberman, J. A. et al. Serotonergic basis of antipsychotic drug effects in schizophrenia. Biol. Psychiatry 44, 1099–1117 (1998)
Miyamoto, S., Duncan, G. E., Marx, C. E. & Lieberman, J. A. Treatments for schizophrenia: a critical review of pharmacology and mechanisms of action of antipsychotic drugs. Mol. Psychiatry 10, 79–104 (2005)
Aghajanian, G. K. & Marek, G. J. Serotonin model of schizophrenia: emerging role of glutamate mechanisms. Brain Res. Brain Res. Rev. 31, 302–312 (2000)
Marek, G. J. Metabotropic glutamate 2/3 receptors as drug targets. Curr. Opin. Pharmacol. 4, 18–22 (2004)
Patil, S. T. et al. Activation of mGlu2/3 receptors as a new approach to treat schizophrenia: a randomized Phase 2 clinical trial. Nature Med. 13, 1102–1107 (2007)
Gonzalez-Maeso, J. et al. Hallucinogens recruit specific cortical 5-HT2A receptor-mediated signaling pathways to affect behavior. Neuron 53, 439–452 (2007)
Gonzalez-Maeso, J. et al. Transcriptome fingerprints distinguish hallucinogenic and nonhallucinogenic 5-hydroxytryptamine 2A receptor agonist effects in mouse somatosensory cortex. J. Neurosci. 23, 8836–8843 (2003)
Vollenweider, F. X., Vollenweider-Scherpenhuyzen, M. F., Babler, A., Vogel, H. & Hell, D. Psilocybin induces schizophrenia-like psychosis in humans via a serotonin-2 agonist action. Neuroreport 9, 3897–3902 (1998)
Gouzoulis-Mayfrank, E. et al. Psychological effects of (S)-ketamine and N,N-dimethyltryptamine (DMT): a double-blind, cross-over study in healthy volunteers. Pharmacopsychiatry 38, 301–311 (2005)
Colpaert, F. C. Discovering risperidone: the LSD model of psychopathology. Nature Rev. Drug Discov. 2, 315–320 (2003)
Marek, G. J., Wright, R. A., Schoepp, D. D., Monn, J. A. & Aghajanian, G. K. Physiological antagonism between 5-hydroxytryptamine2A and group II metabotropic glutamate receptors in prefrontal cortex. J. Pharmacol. Exp. Ther. 292, 76–87 (2000)
Weisstaub, N. V. et al. Cortical 5-HT2A receptor signaling modulates anxiety-like behaviors in mice. Science 313, 536–540 (2006)
Benneyworth, M. A. et al. A selective positive allosteric modulator of metabotropic glutamate receptor subtype 2 blocks a hallucinogenic drug model of psychosis. Mol. Pharmacol. 72, 477–484 (2007)
Angers, S., Salahpour, A. & Bouvier, M. Dimerization: an emerging concept for G protein-coupled receptor ontogeny and function. Annu. Rev. Pharmacol. Toxicol. 42, 409–435 (2002)
Lopez-Gimenez, J. F., Canals, M., Pediani, J. D. & Milligan, G. The α1b-adrenoceptor exists as a higher-order oligomer: effective oligomerization is required for receptor maturation, surface delivery, and function. Mol. Pharmacol. 71, 1015–1029 (2007)
Wright, R. A., Arnold, M. B., Wheeler, W. J., Ornstein, P. L. & Schoepp, D. D. [3H]LY341495 binding to group II metabotropic glutamate receptors in rat brain. J. Pharmacol. Exp. Ther. 298, 453–460 (2001)
Palczewski, K. et al. Crystal structure of rhodopsin: A G protein-coupled receptor. Science 289, 739–745 (2000)
Fotiadis, D. et al. Atomic-force microscopy: Rhodopsin dimers in native disc membranes. Nature 421, 127–128 (2003)
Kenakin, T. Efficacy at G-protein-coupled receptors. Nature Rev. Drug Discov. 1, 103–110 (2002)
Gonzalez-Maeso, J., Rodriguez-Puertas, R. & Meana, J. J. Quantitative stoichiometry of G-proteins activated by μ-opioid receptors in postmortem human brain. Eur. J. Pharmacol. 452, 21–33 (2002)
Carlsson, A. The neurochemical circuitry of schizophrenia. Pharmacopsychiatry 39 (Suppl. 1). S10–S14 (2006)
Vollenweider, F. X. & Geyer, M. A. A systems model of altered consciousness: integrating natural and drug-induced psychoses. Brain Res. Bull. 56, 495–507 (2001)
Vollenweider, F. X. et al. Positron emission tomography and fluorodeoxyglucose studies of metabolic hyperfrontality and psychopathology in the psilocybin model of psychosis. Neuropsychopharmacology 16, 357–372 (1997)
Umbricht, D. et al. Effects of the 5-HT2A agonist psilocybin on mismatch negativity generation and AX-continuous performance task: implications for the neuropharmacology of cognitive deficits in schizophrenia. Neuropsychopharmacology 28, 170–181 (2003)
Gouzoulis-Mayfrank, E. et al. Inhibition of return in the human 5HT2A agonist and NMDA antagonist model of psychosis. Neuropsychopharmacology 31, 431–441 (2006)
Dean, B. The cortical serotonin2A receptor and the pathology of schizophrenia: a likely accomplice. J. Neurochem. 85, 1–13 (2003)
Davidson, M. et al. Severity of symptoms in chronically institutionalized geriatric schizophrenic patients. Am. J. Psychiatry 152, 197–207 (1995)
Gurevich, E. V. & Joyce, J. N. Alterations in the cortical serotonergic system in schizophrenia: a postmortem study. Biol. Psychiatry 42, 529–545 (1997)
Ebersole, B. J., Visiers, I., Weinstein, H. & Sealfon, S. C. Molecular basis of partial agonism: orientation of indoleamine ligands in the binding pocket of the human serotonin 5-HT2A receptor determines relative efficacy. Mol. Pharmacol. 63, 36–43 (2003)
James, J. R., Oliveira, M. I., Carmo, A. M., Iaboni, A. & Davis, S. J. A rigorous experimental framework for detecting protein oligomerization using bioluminescence resonance energy transfer. Nature Methods 3, 1001–1006 (2006)
Blahos, J. et al. Extreme C terminus of G protein α-subunits contains a site that discriminates between Gi-coupled metabotropic glutamate receptors. J. Biol. Chem. 273, 25765–25769 (1998)
Cherezov, V. et al. High-resolution crystal structure of an engineered human β2-adrenergic G protein-coupled receptor. Science 318, 1258–1265 (2007)
Li, J., Edwards, P. C., Burghammer, M., Villa, C. & Schertler, G. F. Structure of bovine rhodopsin in a trigonal crystal form. J. Mol. Biol. 343, 1409–1438 (2004)
Sali, A. & Blundell, T. L. Comparative protein modelling by satisfaction of spatial restraints. J. Mol. Biol. 234, 779–815 (1993)
Binet, V. et al. Common structural requirements for heptahelical domain function in class A and class C G protein-coupled receptors. J. Biol. Chem. 282, 12154–12163 (2007)
Altschul, S. F., Gish, W., Miller, W., Myers, E. W. & Lipman, D. J. Basic local alignment search tool. J. Mol. Biol. 215, 403–410 (1990)
Thompson, J. D., Higgins, D. G. & Gibson, T. J. CLUSTAL W: improving the sensitivity of progressive multiple sequence alignment through sequence weighting, position-specific gap penalties and weight matrix choice. Nucleic Acids Res. 22, 4673–4680 (1994)
Liang, Y. et al. Organization of the G protein-coupled receptors rhodopsin and opsin in native membranes. J. Biol. Chem. 278, 21655–21662 (2003)
Chan, P., Yuen, T., Ruf, F., Gonzalez-Maeso, J. & Sealfon, S. C. Method for multiplex cellular detection of mRNAs using quantum dot fluorescent in situ hybridization. Nucleic Acids Res. 33, e161 (2005)
American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders 4th edn (American Psychiatric Association, Washington DC, 1994)
Preece, P. & Cairns, N. J. Quantifying mRNA in postmortem human brain: influence of gender, age at death, postmortem interval, brain pH, agonal state and inter-lobe mRNA variance. Brain Res. Mol. Brain Res. 118, 60–71 (2003)
Li, J. Z. et al. Systematic changes in gene expression in postmortem human brains associated with tissue pH and terminal medical conditions. Hum. Mol. Genet. 13, 609–616 (2004)
Acknowledgements
We thank L. Devi and L. Ivic for critiquing the manuscript; S. Morgello and the Manhattan HIV Brain Bank for providing control brain cortex; I. Rodil, L. Urigüen and B. Lin for assistance with biochemical assays; the Mount Sinai Microscopy and Microarray, Real-Time PCR and Bioinformatics Shared Research Facilities; the staff members of the Basque Institute of Legal Medicine for their cooperation in the study; J. H. Prather for a gift of LY379268; and J.-P. Pin for providing the signalling peptide sequence of rat mGluR5. This study was supported by the National Institutes of Health, UPV/EHU and the Basque Government, the Spanish Ministry of Health, the REM-TAP Network, the Whitehall Foundation, the Gatsby Foundation and the American Foundation for Suicide Prevention.
Author Contributions J.G.M. and S.C.S. designed experiments, supervised research and wrote the manuscript. J.G.M. performed experiments. R.L.A. performed BRET experiments. T.Y. designed and cloned receptor chimaeras. Y.O. assisted with experiments. P.C. performed FISH studies. N.V.W. and M.Z., supervised by J.A.G., performed behaviour experiments and developed mutant mouse lines. J.L.G., supervised by G.M., performed FRET experiments. M.F. performed computer modelling. L.F.C. and J.J.M. performed schizophrenia post-mortem human brain studies. All authors discussed the results and commented on the manuscript.
Author information
Authors and Affiliations
Corresponding authors
Supplementary information
Supplementary Information
The file contains Supplementary Methods with additional references, Supplementary Tables S1-S12, and Supplementary Figures S1-S14 with Legends. (PDF 12531 kb)
Rights and permissions
About this article
Cite this article
González-Maeso, J., Ang, R., Yuen, T. et al. Identification of a serotonin/glutamate receptor complex implicated in psychosis. Nature 452, 93–97 (2008). https://doi.org/10.1038/nature06612
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nature06612
This article is cited by
-
Therapeutic mechanisms of psychedelics and entactogens
Neuropsychopharmacology (2024)
-
Non-canonical interplay between glutamatergic NMDA and dopamine receptors shapes synaptogenesis
Nature Communications (2024)
-
Psilocybin-induced default mode network hypoconnectivity is blunted in alcohol-dependent rats
Translational Psychiatry (2023)
-
Mechanisms and molecular targets surrounding the potential therapeutic effects of psychedelics
Molecular Psychiatry (2023)
-
Atypical antipsychotics attenuate MK801-induced social withdrawal and hyperlocomotion in the RHA rat model of schizophrenia-relevant features
Psychopharmacology (2023)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.