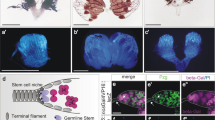
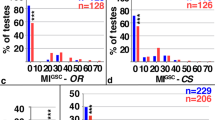
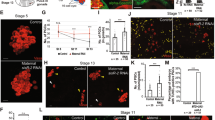
Abstract
The ability of organs such as the liver or the lymphoid system to maintain their original size or regain it after injury is well documented1,2. However, little is known about how these organs sense that equilibrium is breached, and how they cease changing when homeostasis is reached. Similarly, it remains unclear how, during normal development, different cell types within an organ coordinate their growth. Here we show that during gonad development in the fruitfly Drosophila melanogaster the proliferation of primordial germ cells (PGCs) and survival of the somatic intermingled cells (ICs) that contact them are coordinated by means of a feedback mechanism composed of a positive signal and a negative signal. PGCs express the EGF receptor (EGFR) ligand Spitz, which is required for IC survival. In turn, ICs inhibit PGC proliferation. Thus, homeostasis and coordination of growth between soma and germ line in the larval ovary is achieved by using a sensor of PGC numbers (EGFR-mediated survival of ICs) coupled to a correction mechanism inhibiting PGC proliferation. This feedback loop ensures that sufficient numbers of PGCs exist to fill all the stem-cell niches that form at the end of larval development. We propose that similar feedback mechanisms might be generally used for coordinated growth, regeneration and homeostasis.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Freitas, A. A. & Rocha, B. Population biology of lymphocytes: the flight for survival. Annu. Rev. Immunol. 18, 83–111 (2000)
Michalopoulos, G. K. & DeFrances, M. C. Liver regeneration. Science 276, 60–66 (1997)
Gilboa, L. & Lehmann, R. How different is Venus from Mars? The genetics of germ-line stem cells in Drosophila females and males. Development 131, 4895–4905 (2004)
Godt, D. & Laski, F. A. Mechanisms of cell rearrangement and cell recruitment in Drosophila ovary morphogenesis and the requirement of bric a brac. Development 121, 173–187 (1995)
Song, X., Zhu, C. H., Doan, C. & Xie, T. Germline stem cells anchored by adherens junctions in the Drosophila ovary niches. Science 296, 1855–1857 (2002)
Zhu, C. H. & Xie, T. Clonal expansion of ovarian germline stem cells during niche formation in Drosophila. Development 130, 2579–2588 (2003)
Poirié, M., Niederer, E. & Steinmann-Zwicky, M. A sex-specific number of germ cells in embryonic gonads of Drosophila. Development 121, 1867–1873 (1995)
Robertson, S. E., Dockendorff, T. C., Leatherman, J. L., Faulkner, D. L. & Jongens, T. A. germ cell-less is required only during the establishment of the germ cell lineage of Drosophila and has activities which are dependent and independent of its localization to the nuclear envelope. Dev. Biol. 215, 288–297 (1999)
Yohn, C. B., Pusateri, L., Barbosa, V. & Lehmann, R. l(3)malignant brain tumor and three novel genes are required for Drosophila germ-cell formation. Genetics 165, 1889–1900 (2003)
Van Doren, M., Williamson, A. & Lehmann, R. Regulation of zygotic gene expression in Drosophila primordial germ cells. Curr. Biol. 8, 243–246 (1998)
Shilo, B. Z. Signaling by the Drosophila epidermal growth factor receptor pathway during development. Exp. Cell Res. 284, 140–149 (2003)
Nilson, L. A. & Schupbach, T. EGF receptor signaling in Drosophila oogenesis. Curr. Top. Dev. Biol. 44, 203–243 (1999)
Schulz, C., Wood, C. G., Jones, D. L., Tazuke, S. I. & Fuller, M. T. Signaling from germ cells mediated by the rhomboid homolog stet organizes encapsulation by somatic support cells. Development 129, 4523–4534 (2002)
Li, M. A., Alls, J. D., Avancini, R. M., Koo, K. & Godt, D. The large Maf factor Traffic Jam controls gonad morphogenesis in Drosophila. Nature Cell Biol. 5, 994–1000 (2003)
Bergmann, A., Tugentman, M., Shilo, B. Z. & Steller, H. Regulation of cell number by MAPK-dependent control of apoptosis: a mechanism for trophic survival signaling. Dev. Cell 2, 159–170 (2002)
Datar, S. A., Jacobs, H. W., de la Cruz, A. F., Lehner, C. F. & Edgar, B. A. The Drosophila cyclin D-Cdk4 complex promotes cellular growth. EMBO J. 19, 4543–4554 (2000)
Kai, T. & Spradling, A. An empty Drosophila stem cell niche reactivates the proliferation of ectopic cells. Proc. Natl Acad. Sci. USA 100, 4633–4638 (2003)
Margolis, J. & Spradling, A. Identification and behavior of epithelial stem cells in the Drosophila ovary. Development 121, 3797–3807 (1995)
Van Buskirk, C. & Schupbach, T. Versatility in signalling: multiple responses to EGF receptor activation during Drosophila oogenesis. Trends Cell Biol. 9, 1–4 (1999)
Tran, J., Brenner, T. J. & DiNardo, S. Somatic control over the germline stem cell lineage during Drosophila spermatogenesis. Nature 407, 754–757 (2000)
Kiger, A. A., White-Cooper, H. & Fuller, M. T. Somatic support cells restrict germline stem cell self-renewal and promote differentiation. Nature 407, 750–754 (2000)
Roth, S., Neumann-Silberberg, F. S., Barcelo, G. & Schüpbach, T. cornichon and the EGF receptor signaling process are necessary for both anterior-posterior and dorsal-ventral pattern formation in Drosophila. Cell 81, 967–978 (1995)
Gilboa, L. & Lehmann, R. Repression of primordial germ cell differentiation parallels germ line stem cell maintenance. Curr. Biol. 14, 981–986 (2004)
Rodrigues, A. B., Werner, E. & Moses, K. Genetic and biochemical analysis of the role of Egfr in the morphogenetic furrow of the developing Drosophila eye. Development 132, 4697–4707 (2005)
Acknowledgements
We thank J. Treisman for her support with both materials and ideas; G. Dietzl and B. Dickson for sharing UAS-SpiRNAi before publication; B. Edgar and L. Johnston for materials; J. Lafaille for discussions; S. Burden and J. Morris for critical reading of the manuscript; and C. Navarro, D. Siekhaus and all members of the Lehmann laboratory for comments on the manuscript. The Bloomington Stock Center provided reagents. L.G. is supported by a fellowship from the Helen and Martin Kimmel Center for Stem Cell Biology. R.L. is a Howard Hughes Medical Institute investigator.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
Reprints and permissions information is available at www.nature.com/reprints. The authors declare no competing financial interests.
Supplementary information
Supplementary Figure 1
This Supplementary Figure shows the gonadal expression pattern of the protein Traffic Jam during larval development in wild type and in EgfrCA gonads. (PDF 96 kb)
Rights and permissions
About this article
Cite this article
Gilboa, L., Lehmann, R. Soma–germline interactions coordinate homeostasis and growth in the Drosophila gonad. Nature 443, 97–100 (2006). https://doi.org/10.1038/nature05068
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nature05068
This article is cited by
-
Niche formation and function in developing tissue: studies from the Drosophila ovary
Cell Communication and Signaling (2023)
-
Aedes aegypti exhibits a distinctive mode of late ovarian development
BMC Biology (2023)
-
Cyst stem cell lineage eIF5 non-autonomously prevents testicular germ cell tumor formation via eIF1A/eIF2γ-mediated pre-initiation complex
Stem Cell Research & Therapy (2022)
-
A dual role of lola in Drosophila ovary development: regulating stem cell niche establishment and repressing apoptosis
Cell Death & Disease (2022)
-
Maternally inherited intron coordinates primordial germ cell homeostasis during Drosophila embryogenesis
Cell Death & Differentiation (2021)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.