Abstract
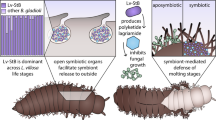
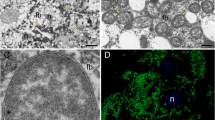
Transmission of obligate bacterial symbionts between generations is vital for the survival of the host. Although the larvae of certain hydrothermal vent tubeworms (Vestimentifera, Siboglinidae) are symbiont-free and possess a transient digestive system, these structures are lost during development, resulting in adult animals that are nutritionally dependent on their bacterial symbionts. Thus, each generation of tubeworms must be newly colonized with its specific symbiont1,2. Here we present a model for tubeworm symbiont acquisition and the development of the symbiont-housing organ, the trophosome. Our data indicate that the bacterial symbionts colonize the developing tube of the settled larvae and enter the host through the skin, a process that continues through the early juvenile stages during which the trophosome is established from mesodermal tissue. In later juvenile stages we observed massive apoptosis of host epidermis, muscles and undifferentiated mesodermal tissue, which was coincident with the cessation of the colonization process. Characterizing the symbiont transmission process in this finely tuned mutualistic symbiosis provides another model of symbiont acquisition and additional insights into underlying mechanisms common to both pathogenic infections and beneficial host–symbiont interactions.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Cary, S. C., Warren, W., Anderson, E. & Giovannoni, S. Identification and localization of bacterial endosymbionts in hydrothermal vent taxa with symbiont-specific polymerase chain reaction amplification and in situ hybridization techniques. Mol. Mar. Biol. Biotechnol. 2, 51–62 (1993)
Di Meo, C. A. et al. Genetic variation among endosymbionts of widely distributed vestimentiferan tubeworms. Appl. Environ. Microbiol. 66, 651–658 (2000)
van Rhijn, P. & Vanderleyden, J. The Rhizobium–plant symbiosis. Microbiol. Rev. 59, 124–142 (1995)
McFall-Ngai, M. J. & Ruby, E. G. Developmental biology in marine invertebrate symbioses. Curr. Opin. Microbiol. 3, 603–607 (2000)
Trench, R. K. Microalgal–invertebrate symbioses: a review. Endocytobiosis Cell Res. 9, 135–175 (1993)
Jones, M. L. & Gardiner, S. L. Evidence for a transient digestive tract in Vestimentifera. Proc. Biol. Soc. Wash. 101, 423–433 (1988)
Southward, E. C. Development of the gut and segmentation of newly settled stages of Ridgeia (Vestimentifera): Implications for relationship between Vestimentifera and Pogonophora. J. Mar. Biol. Ass. UK 68, 465–487 (1988)
Callsen-Cencic, P. & Flügel, H. J. Larval development and the formation of the gut Siboglinum poseidoni Flügel & Langhof (Pogonophora, Perviata), evidence of protostomian affinity. Sarsia 80, 73–89 (1995)
Feldman, R. A., Black, M. B., Cary, C. S., Lutz, R. A. & Vrijenhoek, R. C. Molecular phylogenetics of bacterial endosymbionts and their vestimentiferan hosts. Mol. Mar. Biol. Biotechnol. 6, 268–277 (1997)
Shank, T. M. et al. Temporal and spatial patterns of biological community development at nascent deep-sea hydrothermal vents (9°50′N, East Pacific Rise). Deep-sea Res. II 45, 465–515 (1998)
Cordes, E. E., Arthur, M. A., Shea, K., Arvidson, R. S. & Fisher, C. R. Modeling the mutualistic interactions between tubeworms and microbial consortia. PLoS Biol. 3, e77 (2005)
Rouse, G. W., Goffredi, S. K. & Vrijenhoek, R. C. Osedax: bone-eating marine worms with dwarf males. Science 305, 668–671 (2004)
Southward, E. C., Schulze, A. & Gardiner, S. L. Pogonophora (Annelida): form and function. Hydrobiologia 535/536, 227–251 (2005)
Bright, M. & Sorgo, A. Ultrastructural reinvestigation of the trophosome in adults of Riftia pachyptila (Annelida, Siboglinidae). Invertebr. Biol. 122, 345–366 (2003)
Heimler, H. Larvae. Microfauna Marina 4, 353–371 (1988)
Young, C. M., Vázquez, E., Metaxas, A. & Tyler, P. A. Embryology of vestimentiferan tube worms from deep-sea methane/sulphide seeps. Nature 381, 514–516 (1996)
Marsh, A. G., Mullineaux, L. S., Young, C. M. & Manahan, D. T. Larval dispersal potential of the tubeworm Riftia pachyptila at deep-sea hydrothermal vents. Nature 411, 77–80 (2001)
Vaux, D. Toward an understanding of the molecular mechanisms of physiological cell death. Proc. Natl Acad. Sci. USA 90, 786–789 (1993)
Clarke, P. G. Developmental cell death: morphological diversity and multiple mechanisms. Anat. Embryol. (Berl.) 181, 195–213 (1990)
Twomey, C. & McCarthy, J. V. Pathways of apoptosis and importance in development. J. Cell. Mol. Med. 9, 345–359 (2005)
Zychlinsky, A. & Sansonetti, P. Apoptosis in bacterial pathogenesis. J. Clin. Invest. 100, 493–495 (1997)
Molloy, A., Laochumroonvorapong, P. & Kaplan, G. Apoptosis, but not necrosis, of infected monocytes is coupled with killing of intracellular bacillus Calmette-Guerin. J. Exp. Med. 180, 1499–1509 (1994)
Foster, J. S. & McFall-Ngai, M. J. Induction of apoptosis by cooperative bacteria in the morphogenesis of host epithelial tissues. Dev. Genes Evol. 208, 295–303 (1998)
Mullineaux, L. S., Fisher, C. R., Peterson, C. H. & Schaeffer, S. W. Tubeworm succession at hydrothermal vents: use of biogenic cues to reduce habitat selection error? Oecologia 123, 275–284 (2000)
Dubilier, N., Giere, O., Distel, D. L. & Cavanaugh, C. M. Characterization of chemoautotrophic symbionts in a gutless marine worm (Oligochaeta, Annelida) by phylogenetic 16S rRNA sequence analysis and in situ hybridization. Appl. Environ. Microbiol. 61, 2346–2350 (1995)
Ludwig, W. et al. ARB: a software environment for sequence data. Nucleic Acids Res. 32, 1363–1371 (2004)
Cole, J. R. et al. The Ribosomal Database Project (RDP-II): sequences and tools for high-throughput rRNA analysis. Nucleic Acids Res. 33, D294–D296 (2005)
Benson, D. A. et al. GenBank. Nucleic Acids Res. 27, 12–17 (1999)
Daims, H., Brühl, A., Amann, R., Schleifer, K.-H. & Wagner, M. The domain-specific probe EUB338 is insufficient for the detection of all Bacteria: Development and evaluation of a more comprehensive probe set. Syst. Appl. Microbiol. 22, 434–444 (1999)
Manz, W., Amann, R., Ludwig, W., Wagner, M. & Schleifer, K. H. Phylogenetic oligonucleotide probes for the major subclasses of Proteobacteria: Problems and solutions. Syst. Appl. Microbiol. 15, 593–600 (1992)
Acknowledgements
We thank the captain and crew of the RV Atlantis and the crew of the DSV Alvin for their continuous support; H. Grillitsch for the schematic illustrations; P. Gahleitner for sectioning; W. Klepal for the EM support; S. C. Cary, C. M. Cavanaugh (grants from NOAA and NSF), J. J. Childress and L. S. Mullineaux for their hospitality on cruises; and C. M. Cavanaugh and M. Horn for their comments on the manuscript. This work was supported by grants from the Austrian Science Foundation and the Austrian Academy of Science to M.B., by a grant from the Faculty of Life Sciences, University of Vienna to A.D.N., and a grant from the US National Science Foundation to C.R.F. Author Contributions M.B. was the project leader, designed the TASCs and performed the t.e.m. work; A.D.N. performed the molecular work and was responsible for the data collection; and A.D.N., C.R.F. and M.B. performed the field work and wrote the paper.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
Reprints and permissions information is available at npg.nature.com/reprintsandpermissions. The authors declare no competing financial interests.
Supplementary information
Supplementary Notes
This file contains the Supplementary Methods, Supplementary Table 1, Supplementary Figures and additional references. (PDF 553 kb)
Rights and permissions
About this article
Cite this article
Nussbaumer, A., Fisher, C. & Bright, M. Horizontal endosymbiont transmission in hydrothermal vent tubeworms. Nature 441, 345–348 (2006). https://doi.org/10.1038/nature04793
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1038/nature04793
This article is cited by
-
Geography, not lifestyle, explains the population structure of free-living and host-associated deep-sea hydrothermal vent snail symbionts
Microbiome (2023)
-
Bacterial symbiont diversity in Arctic seep Oligobrachia siboglinids
Animal Microbiome (2023)
-
Distinct genomic routes underlie transitions to specialised symbiotic lifestyles in deep-sea annelid worms
Nature Communications (2023)
-
Endosymbiont population genomics sheds light on transmission mode, partner specificity, and stability of the scaly-foot snail holobiont
The ISME Journal (2022)
-
Complete gammaproteobacterial endosymbiont genome assembly from a seep tubeworm Lamellibrachia satsuma
Journal of Microbiology (2022)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.