Abstract
Hemipterans are known as hosts to bacterial or fungal symbionts that supplement their unbalanced diet with essential nutrients. Among them, scale insects (Coccomorpha) are characterized by a particularly large diversity of symbiotic systems. Here, using microscopic and genomic approaches, we functionally characterized the symbionts of two scale insects belonging to the Eriococcidae family, Acanthococcus aceris and Gossyparia spuria. These species host Burkholderia bacteria that are localized in the cytoplasm of the fat body cells. Metagenome sequencing revealed very similar and highly reduced genomes (<900KBp) with a low GC content (~38%), making them the smallest and most AT-biased Burkholderia genomes yet sequenced. In their eroded genomes, both symbionts retain biosynthetic pathways for the essential amino acids leucine, isoleucine, valine, threonine, lysine, arginine, histidine, phenylalanine, and precursors for the semi-essential amino acid tyrosine, as well as the cobalamin-dependent methionine synthase MetH. A tryptophan biosynthesis pathway is conserved in the symbiont of G. spuria, but appeared pseudogenized in A. aceris, suggesting differential availability of tryptophan in the two host species’ diets. In addition to the pathways for essential amino acid biosynthesis, both symbionts maintain biosynthetic pathways for multiple cofactors, including riboflavin, cobalamin, thiamine, and folate. The localization of Burkholderia symbionts and their genome traits indicate that the symbiosis between Burkholderia and eriococcids is younger than other hemipteran symbioses, but is functionally convergent. Our results add to the emerging picture of dynamic symbiont replacements in sap-sucking Hemiptera and highlight Burkholderia as widespread and versatile intra- and extracellular symbionts of animals, plants, and fungi.
Similar content being viewed by others
Introduction
Many animals are intimately associated with microorganisms that affect various aspects of their biology, including nutrition, development, reproduction, and defense against natural enemies [1,2,3]. As such, symbiotic microorganisms play a fundamental role in the evolution of several groups of insects, promoting range expansions, speciation, and diversification. Insects feeding on plant sap deficient in essential amino acids and other nitrogenous compounds are particularly prone to engage in long-term symbiotic associations [3, 4]. In fact, mutualistic symbioses with microorganisms that supplement their unbalanced diet have allowed sap-feeding insects to adapt to such restricted food, expand into previously inaccessible ecological niches, and subsequently undergo adaptive diversification [5, 6].
Nutritional, heritable symbioses are ubiquitous within scale insects (Insecta, Coccomorpha)—a diverse group of plant sap-sucking hemipterans with economic importance, encompassing almost 8200 species [7]. They are widespread plant pests feeding on phloem sap and living on different parts of plants, including roots, branches, and leaves. Scale insects may be hosting both bacterial (e.g., Tremblaya, Sodalis, Uzinura, Brownia, Burkholderia) and fungal (Ophiocordyceps) nutritional symbionts, as well as facultative associates (such as Rickettsia, Sphingomonas, Wolbachia) whose functions for the hosts’ biology remain unknown [8, 9]. This large taxonomic diversity of microorganisms associated with scale insects, indicating multiple symbiont acquisition and replacement events, makes them a particularly interesting group for studying symbiosis evolution. Results of recent analyses with the use of molecular tools indicate that Flavobacteria (Bacteroidetes) represent the ancestral symbionts of scale insects, which during further evolution, have been lost in some lineages and replaced by other microorganisms that took over their function [8, 10].
The Eriococcidae (felt scales) is one of the neococcoid families, comprising about 560 species[11]. Its phylogeny and classification are still under discussion; however, the 18S rRNA-based phylogeny of scale insects suggests a paraphyletic origin of eriococcids [11, 12]. Previous studies showed that analyses of scale insect symbiotic microorganisms might help resolve relationships within and among Coccoidea families [13, 14]. Results of studies on symbionts of Eriococcidae, although few, also indicate their diversity [15,16,17,18]. Molecular studies revealed that some eriococcids live in mutualistic relationships with heritable bacteria Arsenophonus, Kotejella (in Greenisca brachypodii), and Burkholderia (in Acanthococcus aceris and Gossyparia spuria) [17, 18]. While the symbionts of G. brachypodii are localized intracellularly in specialized organs (bacteriomes), the Burkholderia symbionts of A. aceris and G. spuria stand out among obligatory, bacterial symbionts in hemipterans due to their occurrence in the cytosol of fat body cells, a localization that is reminiscent of the nitrogen-recycling Blattabacterium symbiont in cockroaches and some termites [19, 20]. Apart from the aforementioned bacterial symbionts, the occurrence of intracellular symbionts of fungal origin engaged in nitrogen recycling has been observed in a planthopper, Nilaparvata lugens, and Japanese cicadas [21, 22].
Bacteria belonging to the genus Burkholderia s.l. are widespread in nature and are also diverse regarding their lifestyle, environment, and ecological roles [23,24,25]. Some strains are pathogenic for plants (e.g., Burkholderia gladioli and Burkholderia glumae), humans, and other animals (e.g., Burkholderia pseudomallei) [24]. However, most Burkholderia species are common inhabitants of soil and rhizosphere with non-pathogenic influence on other organisms [26]. Some of them established beneficial, symbiotic associations with various eukaryotes including phytopathogenic and endophytic fungi, different plants, soil amebas, and insects [24, 25]. So far, within insects, mutualistic symbiotic relationships with Burkholderia were reported for phytophagous stinkbugs (Hemiptera, Pentatomomorpha), ants of the genus Tetraponera (Formicidae: Pseudomyrmecinae), Lagrinii beetles (Coleoptera, Tenebrionidae), and the two above-mentioned species of sap-feeding felt scale insects A. aceris and G. spuria (Eriococcidae) [17, 27,28,29]. In all of these insects except for the eriococcids, Burkholderia lives exclusively extracellularly in crypts or pouch-like organs associated with the gut (in stinkbugs and ants), or in accessory glands of the female reproductive system which produce a secretion that is deposited on the egg surface during oviposition, and in specialized cuticular structures in the larvae (in Lagriini beetles). In contrast, the scale insects A. aceris and G. spuria harbor Burkholderia in the cytoplasm of the fat body cells [17]. According to our knowledge, this is so far the only well-documented intracellular localization of Burkholderia symbionts in insects.
The functional role of Burkholderia symbionts for their host insects’ biology varies across different associations, including nutritional benefits, defense against pathogenic fungi and bacteria, resistance to insecticides, and nitrogen fixation [30,31,32]. The genome size of extracellularly localized Burkholderia symbionts ranges from 2.3–8.5 Mb and is generally smaller than the genomes of soil-living Burkholderia [31,32,33], exhibiting intermediate signs of genome reduction that are often observed in vertically transmitted intracellular and also extracellular symbionts [34,35,36,37]. However, information on genomic features and functional roles of intracellular Burkholderia symbionts remain lacking.
To fill this gap, we sequenced the genomes of the intracellularly localized Burkholderia symbionts of the two ericoccid species A. aceris and G. spuria. Our results show that Burkholderia strains associated with scale insects possess highly reduced genomes with few genes involved in housekeeping functions but retained genes responsible for the biosynthesis of essential amino acids and vitamins. These results reveal the smallest known genomes within the genus Burkholderia and uncover a case of a fat-body-localized symbiont functionally replacing bacteriome-localized symbionts in this group of insects.
Materials and methods
Insects
Adult females of maple felt scale, A. aceris, and European elm scale, G. spuria, were collected in Kraków in May and June 2013 and 2015 from branches of maples, Acer platanoides (Sapindaceae Juss.) and elms, Ulmus laevis Pall (Ulmaceae Mirb.), respectively. Both examined species are polyphagous insects feeding on phloem sap. Females of A. aceris live on trees (mainly from the family Sapindaceae), on rose bushes, and on boxwoods (http://scalenet.info/catalogue/Acanthococcus aceris/). Females of G. spuria live on trees (mainly from the family Ulmaceae), on mistletoes (Viscum spp.), and on grasses from the family Poaceae (http://scalenet.info/catalogue/Gossyparia spuria/).
Histological and ultrastructural analyses
Adult females of A. aceris and G. spuria were fixed in 2.5% glutaraldehyde in 0.1 M phosphate buffer (pH 7.4) at 4 °C for a period of three months. The material was then rinsed in the same buffer with the addition of sucrose (5.8 g/100 ml) and postfixed in 1% osmium tetroxide for 1.5 h, dehydrated in a graded series of ethanol and acetone and embedded in epoxy resin Epon 812 (Serva, Heidelberg, Germany). Semithin sections (1 µm thick) were stained in 1% methylene blue in 1% borax, examined, and subsequently photographed under a Nikon Eclipse 80i light microscope (LM). Ultrathin sections (90 nm thick) were contrasted with uranyl acetate and lead citrate and examined and photographed under a Jeol JEM 2100 electron transmission microscope (TEM) at 80 kV.
Symbiont localization by fluorescence in situ hybridization (FISH)
FISH was conducted with a Burkholderia-specific probe (Cy5-GCTCTTGCGTAGCAACTAAG). Females preserved in 100% ethanol were rehydrated, fixed in 4% formaldehyde, and dehydrated through incubations in 80%, 90%, and 100% ethanol and acetone. The material was then embedded in Technovit 8100 resin and cut into sections (1 µm thick). Hybridization was performed using a hybridization buffer containing: 1 M Tris–HCl (pH 8.0), 5 M NaCl, 20% SDS, 30% formamide, and distilled water. The slides were incubated in 200 μl of hybridization solution (hybridization buffer + 10 µM probe) overnight at room temperature [38]. Next, the slides were washed in PBS three times for 10 min, dried, and covered with ProLong Gold Antifade Reagent (Life Technologies). The hybridized slides were then examined using a confocal laser scanning microscope Zeiss Axio Observer LSM 710.
DNA extraction and sequencing
Females of A. aceris and G. spuria destined for molecular analyses were preserved in 100% ethanol. The total genomic DNA was extracted from whole insects using Sherlock AX DNA Extraction kit (A&A Biotechnology) according to the manufacturer’s protocol. Isolated DNA was stored at −20 °C until further analyses. One pooled sample each of A. aceris and G. spuria, respectively, was sent to CeGat (Tübingen) for Illumina library preparation and sequencing. Paired-end libraries were prepared using the Nextera XT DNA Library Prep Kit (Illumina), and 2 × 150 bp PE reads were generated on a NovaSeq 6000 at sequencing depths of 9.8 Gb (A. aceris) and 26.0 Gb (G. spuria), respectively.
Symbiont genome assembly and annotation
All computational analyses were performed using the KBase platform [39]. Sequence quality was assessed and trimmed with Trimmomatic v0.36 using default parameters [40]. Subsequently, sequencing reads were assembled to contigs with MEGAHIT v1.2.9 [41] for each insect species, respectively. To identify and filter sequences specific to the microbial symbionts, binning methods MetaBAT2 v1.7 [42] and MaxBin2 v2.2.4 [43] were used in combination with DASTool v1.1.2 [44] to cluster the contigs in several bins. The quality and taxonomic identity of those bins were then assessed with CheckM v1.0.18 [45]. For further analyses, the bins that were annotated as genus Burkholderia were used for both species. Since both extracted symbiont genomes were closely related to each other, the isolated symbiont genome of A. aceris was used as a reference to align and arrange the contigs of the G. spuria symbiont genome. Both symbiont genomes were then annotated using RAST [46], of which the annotated and called genes were used in further analyses.
Phylogeny based on Burkholderia genomes
To check the taxonomic identity and phylogenetic context of the recovered Burkholderia symbionts, a phylogenetic tree was calculated by taking into account whole genome information. Potential Burkholderia reference species were selected based on a recent phylogenetic study of Burkholderia species [47]. These reference genomes were then annotated in KBase [39] with RAST [46]. Within KBase, a multiple sequence alignment was performed based on a conserved set of 49 COG (Clusters of Orthologous Groups) family domains, respectively. The alignments were concatenated, and an approximately maximum likelihood tree was calculated using FastTree2 v2.1.9 [48] with 1000 resamples for local branch support values. The resulting tree was then exported in Newick format and edited with MEGA11 v11.0.10 [49].
Comparative genomics of related symbionts
To pinpoint the putative metabolic function of the recovered Burkholderia symbionts, their metabolic capabilities were put in context with other insect symbionts using a comparative genomics approach. For this, automatic gene annotations of the Burkholderia symbionts and other relevant hemipteran symbionts (Fig. S1) were assigned to COGs (Clusters of Orthologous Groups) within KBase [39]. Since most symbionts of our set are involved in co-enzyme and amino acid metabolism, genes of those metabolic categories were extracted and then compared based on their presence and absence in the different genomes. The results were then displayed in a heatmap using the R package ComplexHeatmap [50].
Results
Localization of Burkholderia symbionts in A. aceris and G. spuria
As reported previously [17], rod-shaped Burkholderia bacteria occur intracellularly in the cytosol of fat body cells of A. aceris and G. spuria (Fig. 1A–C). The presence of high densities of symbiont cells in the fat body cells was confirmed by means of fluorescence in situ hybridization with a Burkholderia-specific probe (Fig. 1D). Transmission electron microscopy confirmed the presence of three membranes surrounding the Burkholderia cells (two bacterial membranes and one symbiosome membrane) (Fig. 1C), supporting previous observations describing the eriococcid symbiosis as the first documented intracellular association between insects and Burkholderia [17].
A–D Burkholderia cells in the cytoplasm of the fat body cells of G. spuria, fb—fat body, n—fat body cell nucleus, arrows—Burkholderia cells. A Light microscopy, scale bar—10 µm, B, C Transmission electron microscopy, scale bar—1 µm, D Confocal microscopy, green - Burkholderia cells, scale bar—10 µm.
Genome characteristics
Illumina paired-end sequencing of shotgun libraries from total DNA of A. aceris and G. spuria resulted in 139 and 177 million raw reads, respectively, that were quality checked and trimmed and subsequently assembled into 20,613 (11,843,469 bp) and 78,051 (264,520,377 bp) contigs using Megahit v1.2.9 [41] as implemented in KBase [39]. Binning of contigs with MetaBAT2 v1.7 [42] and MaxBin2 v2.2.4 [43] and combining the results with DASTool v1.1.2 [44] and subsequent reassembly in Geneious v. 2019.1.3 yielded three contigs with 866,825 bp taxonomically assigned to the genus Burkholderia for A. aceris and six for G. spuria. Synteny analyses with Clinker [51] and Nucmer [52] revealed a high degree of similarity and perfect synteny between the symbiont genomes of the two host species, based on which the G. spuria contigs could be easily arranged into three scaffolds by comparison with the A. aceris contigs. Repeat regions at the ends of the contigs and scaffolds prevented assembly into circular chromosomes.
The draft genome of the G. spuria symbiont exhibited a size of 870,232 bp, a G + C content of 37.4% and encoded for 726 CDS after RAST annotation [46] in KBase [39]. CheckM v1.0.18 [45] predicted a completeness of 42.4%, but the annotation of 39 tRNAs for all 20 amino acids indicates that the genome is in fact almost complete. Low estimates of completeness based on searches for conserved single-copy marker genes were expected, since the investigated symbionts have experienced genome erosion, resulting in the loss of many genes that are otherwise conserved across taxonomically related Burkholderia reference genomes. Similarly, the A. aceris symbiont draft genome was 866,825 bp in size, with a G + C content of 37.5%, 748 predicted CDS, an estimated completeness of 39.7%, and 38 tRNAs annotated for 19 amino acids. tRNA-Phe was missing in the original annotation but could be manually annotated based on the comparison with the G. spuria symbiont genome, resulting in 39 tRNAs for all 20 amino acids (Fig. 2). Furthermore, based on the coverage and binning approach, no other contigs could be found in the metagenome that would correspond to the genome of the symbionts. Additionally, the symbiont contigs were flanked by repeat regions (Fig. 2), which are generally difficult to sequence, further indicating that the recovered genomes are indeed complete.
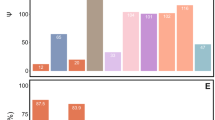
A, B Circular diagrams of Burkholderia symbiont genomes from A G. spuria and B A. aceris. C Size and GC content plot for sequenced genomes of Burkholderia sensu lato strains, highlighting the Eriococcidae symbiont genomes as the smallest and most AT-biased genomes among all Burkholderia s.l. sequenced to date. Colors highlight genus-level affiliation (Burkholderia—blue, Paraburkholderia—gray, Caballeronia—violet) and host associations (fungi—brown, plants—green, insects—red), respectively, and shapes denote draft (triangles) and complete genomes (circles).
Functional potential of the Burkholderia symbionts in eriococcids
The genomes of the two eriococcid symbionts show a high degree of similarity in their functional gene content (Fig. 3). Despite their severely reduced genomes, both retain complete biosynthetic pathways for the essential amino acids leucine, isoleucine, valine, threonine, lysine, arginine, histidine (except for hisB), and phenylalanine, as well as precursors for the semi-essential amino acid tyrosine. A complete biosynthetic pathway for tryptophan biosynthesis is encoded in the G. spuria symbiont genome, whereas trpC, trpD, and trpF were disrupted by frameshift mutations in the A. aceris symbiont genome. Both symbionts are lacking the methionine biosynthesis genes metA, metB, and metC, but encode the cobalamin-dependent methionine synthase MetH (Fig. 3A).
In addition to the pathways for essential amino acid biosynthesis, both symbionts appear to be capable of synthesizing multiple cofactors, including riboflavin (vitamin B2), cobalamin (vitamin B12), and folate (vitamin B9). The G. spuria symbiont additionally encodes pathways for heme and thiamine biosynthesis (vitamin B1) that are lacking from the A. aceris symbiont genome. Furthermore, the symbiont of G. spuria, but not that of A. aceris, encodes multiple transporters as well as enzymes involved in the uptake and assimilatory reduction of sulfate (not shown).
Comparisons with the functional potential of other hemipteran obligate and co-obligate symbionts reveal broad commonalities in essential amino acid biosynthetic capabilities (Fig. S1). By contrast, the distribution of B vitamin biosynthesis pathways across symbionts is patchier, with Baumannia cicadellinicola being the most versatile B vitamin producer. However, the symbionts of A. aceris and G. spuria stand out in their ability to synthesize cobalamin, a pathway that is lacking in most other hemipteran symbionts, with the exception of Hodgkinia cicadicola [53] and Evansia muelleri [54]. Concordantly, Hodgkinia, Evansia, and the Burkholderia symbionts of eriococcids all encode the cobalamin-dependent methionine synthase MetH, instead of the cobalamin-independent version MetE that is encoded by most other symbionts. Thus, it appears likely that cobalamin biosynthesis is only required to provide the cofactor for MetH.
Phylogenetic affiliation of the eriococcid symbionts
Phylogenomic analyses of the A. aceris and G. spuria symbionts and other sequenced Burkholderiales based on 49 COG family domains reveal their affiliation with the genus Burkholderia s.str. (Fig. 4). This is insofar interesting as most other insect-associated Burkholderia s.l. fall into the genera Caballeronia and Paraburkholderia [25], with only the defensive symbionts of Lagria beetles [27, 31, 32] and some environmentally acquired gut symbionts in two hemipteran species Cavelerius saccharivorus and Blissus insularis being affiliated with Burkholderia s.str. [55]. The monophyly of the two eriococcid symbionts and the long branch separating them from the other Burkholderia species, as well as their high degree of synteny, strongly indicate a common ancestry of the A. aceris and G. spuria symbionts and a shared history of genome erosion that predated the split of the two eriococcid host species. Most of the closest relatives are plant or animal pathogens, suggesting a pathogenic ancestry of the symbionts.
The phylogeny was constructed based on 49 COG family domains showing the relationships among Burkholderia strains. Different colors indicate ecological differences (environmental, pathogenic, symbiotic). Filled circles on the nodes represent branch support values above 90% from an approximately maximum likelihood reconstruction using FastTree2 v2.1.9 [48] with 1000 resamples.
Discussion
Bacteria of the genus Burkholderia show a broad spectrum of interactions with other organisms - from pathogenic to mutualistic [23,24,25, 28]. Here, we demonstrate that a strain of Burkholderia established a nutritional intracellular symbiosis with the two eriococcid species A. aceris and G. spuria. Our genomic analyses revealed that these symbionts have the smallest and most AT-biased genomes of any Burkholderia sequenced so far, indicating genome erosion that is typical for long-term associations. However, despite this limitation and their localization outside of bacteriomes, the Burkholderia symbionts in eriococcids fulfill their nutritional function providing host insects with almost all essential amino acids and several B vitamins.
Burkholderia—a new intracellular symbiont of insects
Bacteria from the genus Burkholderia are widespread as extracellular symbionts in insects. They usually occur in the midgut crypts (in stinkbugs and bean bugs) or special dorsal organs (in beetles) and fulfill nutritional, detoxifying, or defensive roles [27, 56]. For example, in Lagria species, Burkholderia produces bioactive secondary metabolites that protect the beetles’ eggs and larvae from microbial infections [27], while in the bean bug Riptortus pedestris the symbiont recycles host metabolic wastes [56] and detoxifies pesticides [57]. To our knowledge, eriococcids with Burkholderia cells localized within the cytoplasm of fat body cells present the only well-documented example of an intracellular heritable symbiosis with these bacteria in insects [17]. While Ishii et al. [58] detected Burkholderia in bacteriome and ovary samples of the leafhopper Macrosteles striifrons by PCR-based methods, the exact localization, as well as ultrastructure of these microorganisms, remain unknown due to the lack of microscopic observations. In fact, the authors of the study carefully conclude that Burkholderia ‘may be either an environmental contaminant or a gut microbe, but […] the possibility that it may represent a previously unknown type of vertically transmitted Burkholderia endosymbiont cannot be ruled out, and deserves future experimental verification’. However, it is worth mentioning that a species of the genus Mycetohabitants (Candidatus Mycetohabitans vallotii) belonging to Burkholderia s.l. has been identified in the cytoplasm of the bacteriocytes of adelgids from the genus Adelges [59]. Outside the metazoans, intracellular symbionts representing Burkholderia s.l. (formerly classified as Burkholderia s.str.) have been detected in the plant pathogenic fungus Rhizopus sp. (bacterium Mycetohabitans) and in amoebae (bacterium Paraburkholderia) [60,61,62,63]. In both cases, the host benefits from the bacterial symbiont’s biosynthetic capacities to obtain nutrients. In the soil ameba Dictyostelium discoideum, the Paraburkholderia symbiont does not provide nutrients directly to the host cell, but it induces co-infections with secondary bacteria that then serve as a food source [64]. By contrast, Mycetohabitans rhizoxinica symbionts in the pathogenic fungus Rhizopus are responsible for the production of rhizoxin - an antimitotic toxin that kills the host plant of the fungus, allowing the latter to obtain nutrients from the decaying plant [60]. Both above-mentioned symbioses are not completely closed, as these symbionts can be acquired through vertical and horizontal transmission routes [60, 62]. Intracellular localization of horizontally transmitted symbionts requires the machinery for host cell invasion. Concordantly, bacteria that are prone to establish symbiotic interactions with a host usually utilize the infection mechanisms known for bacterial pathogens involving type 2 or type 3 secretion systems (T2SS/T3SS) during the colonization of host cells and tissues. The role of T3SS in establishing an intracellular symbiosis was described previously in Sodalis-allied bacteria, which are known as widespread insect symbionts [65, 66]. For example, the T3SS in Sodalis pierantonius is likely to mediate infection of midgut epithelial stem cells that develop into adult bacteriomes during the metamorphosis of the cereal weevil Sitophilus oryzae [67]. Also, Partida-Martinez and Hertweck [60] showed that Mycetohabitans living in the cytoplasm of Rhizopus fungi possess T2SS genes that are involved in the secretion of lytic enzymes allowing bacteria to penetrate the fungal cell wall and invade the host cells [67]. Our analyses revealed no evidence for T2SS or T3SS genes in the Burkholderia symbionts of G. spuria and A. aceris, indicating that the Burkholderia symbionts in eriococcids permanently adapted to the vertical transmission and intracellular lifestyle.
Burkholderia symbionts in eriococcids show higher nutritional capacity than symbionts of other sap-sucking hemipterans
Obligate symbioses in hemipterans feeding on unbalanced diets are diverse and involve various bacterial taxa [68]. However, all of them show a similar functional pattern— symbionts supplement the host insect with limited essential amino acids and vitamins [4, 68]. While some hemipteran lineages host only one obligate symbiont (for example mealybugs from Phenacoccinae subfamily hosting only Tremblaya phenacola, or some aphids harboring Buchnera aphidicola), in others the synthesis of amino acids and vitamins is partitioned between two or even more microorganisms with complementary metabolic pathways [4]. This metabolic complementarity between symbionts is common in Auchenorrhyncha, as they usually harbor at least two co-obligatory symbionts [69,70,71]. Our genomic analyses revealed that Burkholderia symbionts in eriococcids lack a co-obligate symbiont and have a higher nutritional capacity in comparison to other hemipteran symbionts, retaining the complete biosynthesis pathways for eight essential amino acids plus precursors for tyrosine (additionally, Burkholderia associated with G. spuria can synthesize tryptophan, whereas Burkholderia in A. aceris cannot). However, both symbionts lack a complete gene set essential for the synthesis of methionine. Only the gene metH encoding cobalamin-dependent methionine synthases is present in both genomes, whereas the remaining genes metC, metB, and metA, responsible for the transformation of homoserine into homocysteine, are missing. It is possible that Burkholderia in eriococcids obtain homocysteine from cysteine through the transsulfuration pathway as they are predicted to synthesize cysteine. The presence of metH is not common among bacterial symbionts insects, and most of them are not able to synthesize cobalamin, which is not required by host insects [72]. Therefore, most insect symbionts instead encode metE – the cobalamin-independent methionine synthase. The exceptions are Hodgkinia and Evansia, which, similarly to the eriococcids’ Burkholderia, are capable of producing cobalamin and encode metH [53, 54]. The lack of some genes involved in methionine biosynthesis is common in symbionts and has been described before in Buchnera, Tremblaya, Carsonella, and Vidania [71, 73,74,75]. Hansen and Moran [73] showed that in aphids, Buchnera and its host cooperate in the production of methionine, with products of host genes completing the missing steps in methionine biosynthesis.
The only difference between the genomes of the two Burkholderia strains in essential amino acids biosynthesis pathways is related to tryptophan biosynthesis. In contrast to G. spuria, in Burkholderia of A. aceris trpC, trpD, and trpF genes were disrupted by frameshift mutations (Fig. S2). This may indicate a recent pseudogenization event or reflect suboptimal expression through transcriptional slippage, as has been observed in other AT-rich endosymbionts [76]. This process would allow for maintaining the functionality of the trp operon despite the presence of apparent frameshift mutations, in line with the observation that most plant-feeders including planthoppers, scale insects, and aphids preserve functional tryptophan-biosynthetic pathways [71, 77, 78]. However, we only observe frameshift mutations in the trp genes and not in other metabolic pathways, suggesting that the symbionts are indeed in the process of losing a functional tryptophane biosynthetic pathway. Additionally, biosynthesis of tryptophan is one of the most expensive among essential amino acids, and therefore its synthesis is commonly outsourced to co-obligate symbionts, indicating that it is probably one of the pathways most rapidly lost when there is an alternative source for this amino acid [4]. Such potential losses of individual biosynthetic pathways took place in other symbioses as well and are usually presumed to be correlated with switches to diets that contain the missing nutrient [37, 79]. As we did not detect any other microbes associated with A. aceris that could supplement the host’s diet with tryptophan, we hypothesize that the insects obtain this amino acids in sufficient amounts from the phloem sap of the host plant A. platanoides.
In addition to the pathways for essential amino acid biosynthesis, both symbionts are capable of synthesizing nonessential amino acids, including serine, cysteine, proline, and glycine. As these nutrients can be produced by host insects, the genes involved in their synthesis have not been conserved in highly specialized symbionts like Sulcia, Carsonella or Tremblaya, with the only exception being Buchnera of the aphid Brevicoryne brassicae, which is predicted to produce cysteine (CP034882). However, some of the additional symbionts that complement the ancient ones, including Purcelliella in planthoppers and Baumannia in sharpshooters, also encode the complete biosynthetic pathway for cysteine, whereas, in other additional symbionts, only single genes were retained [69, 80]. The presence of complete sets of genes involved in the synthesis of four nonessential amino acids in Burkholderia symbiont genomes of ericoccids indicates that these symbiosis are likely younger than other hemipteran symbioses.
Besides amino acids, the Burkholderia symbionts of A. aceris and G. spuria provide cobalamin, riboflavin, thiamine, and folate to the host insect. Such a wide range of B vitamin biosynthetic pathways is unusual among bacterial symbionts of hemipterans, as most of them produce no or only one B vitamin (e.g., cobalamin - Hodgkinia and Evansia, or riboflavin - Buchnera) [53, 54]. However, the record holder in this respect is Baumannia cicadellinicola encoding biosynthesis pathways for eight B vitamins, including thiamine, riboflavin, niacin, pantothenic acid, pyridoxine, biotin, and folic acid [69].
Burkholderia replaced the ancestral symbiont of scale insects
In Hemiptera, repeated symbiont replacements have led to a large diversity and variability of symbiotic systems [6, 22, 81]. Concordantly, the evolutionary history of scale insect symbioses is shaped by multiple symbiont replacements [8, 16, 78, 82, 83]. According to Rosenblueth and co-workers [8], Flavobacteria were the likely ancestral symbionts of scale insects since the origin of this insect taxon in the Cretaceous period, and they have been replaced by other microorganisms at least three times in the evolution of Coccomorpha, i.e. in species of the families Putoidae, Pseudococcidae and Dactylopidae. Previous studies on symbionts in the family Eriococcidae [17, 18] revealed that they may host both gammaproteobacterial (Kotejella and Arsenophonus) and betaproteobacterial (Burkholderia) symbionts, suggesting additional replacement events of Flavobacteria in the evolution of scale insects. This hypothesis is supported by the results of our genomic studies of Burkholderia symbionts in eriococcids A. aceris and G. spuria, indicating that these symbioses are very likely younger than some of the other hemipteran symbioses. With sizes of the approx. 900 Kb, the genomes of Burkholderia symbionts in examined eriococcids are much smaller than genomes of free-living Burkholderia strains (5.5–11.5 Mb) and extracellular Burkholderia symbionts in insects (2.3–8.4 Mb) and, in comparison to them, are characterized by much lower GC contents (app. 37%) [32, 33]. On the other hand, they are larger than genomes of other intracellular, heritable hemipteran symbionts, including both ancient ones like Sulcia (up to 270 Kb) or Carsonella (app. 170 Kb), as well as more recently acquired additional symbionts, e.g., Purceliella (app. 480 Kb) or Baumannia (app. 680 Kb) [69, 71, 75]. Moreover, their nutritional capacities stand out from other symbionts as they produce almost all essential amino acids, some nonessential ones, and four B vitamins. Thus, taking into account the high degree of similarity between Burkholderia strains associated with the two examined eriococcids species and the significant reduction of their genomes that nevertheless retain a broad spectrum of nutritional capacities, we conclude that the symbiosis with Burkholderia in Eriococcidae is a result of a symbiont replacement event that occurred before the split of the genera A. aceris and G. spuria. However, the lack of genomic data for other Eriococcidae species precludes inferences on the distribution and evolution of Burkholderia symbionts in this family.
The Burkholderia localization provides additional evidence for this symbiosis being younger than other symbioses in scale insects, as they do not exhibit the typical localization in bacteriocytes, but are present in the cytoplasm of fat body cells. Bacteriocyte-localized symbionts are pervasive in Sternorrhyncha and Auchenorrhyncha [9, 70, 71, 81, 84, 85]. The limited data about eriococcids symbioses suggest that these insects ancestrally contained bacteriomes, as the gammaproteobacterial symbionts of G. brachypodii - another representative of Eriococcidae family are also bacteriocyte-associated [18]. Thus, the loss of bacteriocytes in the examined eriococcids probably took place upon the acquisition of the Burkholderia symbiont. A similar loss of symbiont-containing organs has been observed in some cicadas, Deltocephalinae leafhoppers, and planthoppers, in which one or both of the bacteriome-associated symbionts (Hodgkinia in cicadas, Nasuia in Deltocephalinae leafhoppers, both Vidania and Sulcia or only Sulcia in planthoppers) have been replaced with a fat body-localized Ophiocordyceps fungus that evolved from a pathogenic ancestor [22, 81]. It is possible that Burkholderia symbionts of eriococcids have experienced a similar evolutionary history, as our phylogenomic analysis indicates that they fall into the (mostly) pathogenic Burkholderia s.str. clade. Symbiont replacements are common among hemipterans [78, 86,87,88,89], and they may allow the hosts to escape the rabbit hole of degenerative symbiont genome evolution [87]. The newly acquired symbionts (e.g. gammaproteobacteria Arsenophonus and Sodalis or Ophiocordyceps-allied fungi) initially possess large genomes, which likely makes them more effective in their nutritional functions [90]. However, after the replacement, functional convergence of symbiont-encoded traits and genome erosion involving reduction of genome size and AT bias usually occur [90].
The change in the symbiont localization from bacteriocytes into fat body cells likely influenced the communication and integration of host-symbiont interaction. As opposed to bacteriomes that are specialized in maintaining and regulating symbionts, fat body cells serve additional purposes, e.g., the production of hydrocarbons and fatty acids, vitellogenins, as well as immune effectors [91]. Therefore, the lack of bacteriocytes and localization in fat body tissue probably affected the evolution of the symbiosis and the regulation of the symbionts by the host. The key to testing hypotheses concerning the evolution of symbiosis will be the comparative analysis of symbioses involving taxonomically different symbiotic microorganisms showing the same localization patterns: Burkholderia in Eriococcidae, Ophiocordyceps in cicadas, planthoppers and aphids, and Blattabacterium in cockroaches.
‘Candidatus Burkholderia endosymbiotica sp. nov.’
As Burkholderia symbionts of A. aceris and G. spuria represent the only insect-associated intracellularly localized Burkholderia strains, we propose to name them ‘Candidatus Burkholderia endosymbiotica sp. nov.’, and denote the host affiliation with AACE and GSPU, respectively (i.e., ‘Candidatus Burkholderia endosymbiotica AACE’, and ‘Candidatus Burkholderia endosymbiotica GSPU’). Phylogenetic position: Pseudomonadota, Burkholderiales, Burkholderiaceae; Gram-negative bacteria, uncultured obligate intracellular symbionts of eriococcids: A. aceris and G. spuria (Hemiptera, Coccomorpha: Eriococcidae); shape and size: rod-shaped bacteria, approximately 2 µm in length and 1 µm in diameter; localization: cytoplasm of fat body cells; role in host biology: nutritional symbionts (providing essential amino acids and B vitamins); inherited vertically (transovarially) between generations; described based on genomes deposited in the NCBI database under Bioproject PRJNA981321 (other sequences deposited in the NCBI belonging to this species: KR904884 and KR904885).
Conclusions
Symbiont acquisitions and replacements represent important driving forces of insects’ evolution and diversification. Establishing symbiosis with ‘new’ microorganisms of large biosynthetic capacities may drastically increase the fitness of host insects and allow them to adapt to novel ecological niches. The obligatory, heritable, nutritional symbiosis between eriococcids and Burkholderia offers new insights into the diversity of beneficial intracellular symbionts that can be recruited by insects and provide additional evidence that host-beneficial bacteria often have a pathogenic origin. Observed changes in symbiont localization, together with functional convergence, in the future may provide valuable insights into the flexibility but also constraints on symbiont metabolism and host control.
Data availability
Sequence data have been deposited in GenBank under Bioproject PRJNA981321. Raw data have been deposited on the MPG Edmond repository (https://edmond.mpdl.mpg.de/) under the link https://doi.org/10.17617/3.3CEYBM.
References
Stouthamer R, Breeuwer JAJ, Hurst GDD. Wolbachia pipientis: microbial manipulator of arthropod reproduction. Annu Rev Microbiol. 1999;53:71–102.
Koropatnick TA, Engle JT, Apicella MA, Stabb EV, Goldman WE, McFall-Ngai MJ. Microbial factor-mediated development in a host-bacterial mutualism. Science. 2004;306:1186.
Baumann P. Biology of bacteriocyte-associated endosymbionts of plant sap-sucking insects. Annu Rev Microbiol. 2005;59:155–89.
Douglas AE. How multi-partner endosymbioses function. Nat Rev Microbiol. 2016;14:731–43.
Janson EM, Stireman JO III, Singer MS, Abbot P. Phytophagous insect-microbe mutualisms and adaptive evolutionary diversification. Evolution. 2008;62:997–1012.
Sudakaran S, Kost C, Kaltenpoth M. Symbiont acquisition and replacement as a source of ecological innovation. Trends Microbiol. 2017;25:375–90.
García Morales M, Denno BD, Miller DR, Miller GL, Ben-Dov Y, Hardy NB. ScaleNet: a literature-based model of scale insect biology and systematics. Database. 2016. https://doi.org/10.1093/database/bav118.
Rosenblueth M, Martínez-Romero J, Ramírez-Puebla ST, Vera-Ponce de León A, Rosas-Pérez T, Bustamante-Brito R. Endosymbiotic microorganisms of scale insects. Rev Espec Cienc Quím Biol. 2018;21:53–69.
Szklarzewicz T, Michalik A, Michalik K. The diversity of symbiotic systems in scale insects. In: Kloc M, editor. Symbiosis: cellular, molecular, medical and evolutionary aspects. Cham: Springer International Publishing; 2020. p. 469–95.
Koteja J. Essay on the prehistory of the scale insects (Homoptera, Coccinea). Ann Zool. 1985;38:461–503.
Gullan PJ, Cook LG. Phylogeny and higher classification of the scale insects (Hemiptera: Sternorrhyncha: Coccoidea). Zootaxa. 2007;1668:413–25.
Cook LG, Gullan PJ, Trueman HE. A preliminary phylogeny of the scale insects (Hemiptera: Sternorrhyncha: Coccoidea) based on nuclear small-subunit ribosomal DNA. Mol Phylogenet Evol. 2002;25:43–52.
Szklarzewicz T, Kalandyk-Kołodziejczyk M, Michalik K, Jankowska W, Michalik A. Symbiotic microorganisms in Puto superbus (Leonardi, 1907) (Insecta, Hemiptera, Coccomorpha: Putoidae). Protoplasma. 2018;255:129–38.
Szklarzewicz T, Kalandyk-Kołodziejczyk M, Michalik A. Ovary structure and symbiotic associates of a ground mealybug, Rhizoecus albidus (Hemiptera, Coccomorpha: Rhizoecidae) and their phylogenetic implications. J Anat. 2022;241:860–72.
Walczuch A. Studien an Coccidensymbionten. Z Morph Ökol Tiere. 1932;25:623–729.
Rosenblueth M, Sayavedra L, Sámano-Sánchez H, Roth A, Martínez-Romero E. Evolutionary relationships of flavobacterial and enterobacterial endosymbionts with their scale insect hosts (Hemiptera: Coccoidea). J Evol Biol. 2012;25:2357–68.
Michalik K, Szklarzewicz T, Kalandyk-Kołodziejczyk M, Jankowska W, Michalik A. Bacteria belonging to the genus Burkholderia are obligatory symbionts of the eriococcids Acanthococcus aceris Signoret, 1875 and Gossyparia spuria (Modeer, 1778) (Insecta, Hemiptera, Coccoidea). Arthropod Struct Dev. 2016;45:265–72.
Michalik A, Schulz F, Michalik K, Wascher F, Horn M, Szklarzewicz T. Coexistence of novel gammaproteobacterial and Arsenophonus symbionts in the scale insect Greenisca brachypodii (Hemiptera, Coccomorpha: Eriococcidae). Environ Microbiol. 2018;20:1148–57.
Bandi C, Sironi M, Damiani G, Magrassi L, Nalepa CA, Laudani U, et al. The establishment of intracellular symbiosis in an ancestor of cockroaches and termites. Proc R Soc Lond B Biol Sci. 1995;259:293–9.
Noda T, Okude G, Meng X-Y, Koga R, Moriyama M, Fukatsu T. Bacteriocytes and Blattabacterium endosymbionts of the German cockroach Blattella germanica, the forest cockroach Blattella nipponica, and other cockroach species. Zool Sci. 2020;37:399–410.
Sasaki T, Kawamura M, Ishikawa H. Nitrogen recycling in the brown planthopper, Nilaparvata lugens: Involvement of yeast-like endosymbionts in uric acid metabolism. J Insect Physiol. 1996;42:125–9.
Matsuura Y, Moriyama M, Łukasik P, Vanderpool D, Tanahashi M, Meng X-Y, et al. Recurrent symbiont recruitment from fungal parasites in cicadas. Proc Natl Acad Sci USA. 2018;115:E5970.
Coenye T, Vandamme P. Diversity and significance of Burkholderia species occupying diverse ecological niches. Environ Microbiol. 2003;5:719–29.
Compant S, Nowak J, Coenye T, Clément C, Ait Barka E. Diversity and occurrence of Burkholderia spp. in the natural environment. FEMS Microbiol Rev. 2008;32:607–26.
Kaltenpoth M, Flórez LV. Versatile and dynamic symbioses between insects and Burkholderia bacteria. Annu Rev Entomol. 2020;65:145–70.
Viallard V, Poirier I, Cournoyer B, Haurat J, Wiebkin S, Ophel-Keller K, et al. Burkholderia graminis sp. nov., a rhizospheric Burkholderia species, and reassessment of [Pseudomonas] phenazinium, [Pseudomonas] pyrrocinia and [Pseudomonas] glathei as Burkholderia. Int J Syst Evol Microbiol. 1998;48:549–63.
Flórez LV, Kaltenpoth M. Symbiont dynamics and strain diversity in the defensive mutualism between Lagria beetles and Burkholderia. Environ Microbiol. 2017;19:3674–88.
Takeshita K, Kikuchi Y. Riptortus pedestris and Burkholderia symbiont: an ideal model system for insect–microbe symbiotic associations. Res Microbiol. 2017;168:175–87.
Wierz JC, Gaube P, Klebsch D, Kaltenpoth M, Flórez LV. Transmission of bacterial symbionts with and without genome erosion between a beetle host and the plant environment. Front Microbiol. 2021;12:715601.
Van Borm S, Buschinger A, Boomsma JJ, Billen J. Tetraponera ants have gut symbionts related to nitrogen–fixing root–nodule bacteria. Proc R Soc Lond B Biol Sci. 2002;269:2023–7.
Flórez LV, Scherlach K, Gaube P, Ross C, Sitte E, Hermes C, et al. Antibiotic-producing symbionts dynamically transition between plant pathogenicity and insect-defensive mutualism. Nat Commun. 2017;8:15172.
Flórez LV, Scherlach K, Miller IJ, Rodrigues A, Kwan JC, Hertweck C, et al. An antifungal polyketide associated with horizontally acquired genes supports symbiont-mediated defense in Lagria villosa beetles. Nat Commun. 2018;9:2478.
Nazir R, Hasem M, Sørensen S, van Elsas JD. Draft genome sequence of the soil bacterium Burkholderia terrae strain BS001, which interacts with fungal surface structures. J Bacteriol. 2012;194:4480–1.
Hosokawa T, Kikuchi Y, Nikoh N, Shimada M, Fukatsu T. Strict host-symbiont cospeciation and reductive genome evolution in insect gut bacteria. PLoS Biol. 2006;4:e337–e337.
Kikuchi Y, Hosokawa T, Nikoh N, Meng X-Y, Kamagata Y, Fukatsu T. Host-symbiont co-speciation and reductive genome evolution in gut symbiotic bacteria of acanthosomatid stinkbugs. BMC Biol. 2009;7:2.
McCutcheon JP, Moran NA. Extreme genome reduction in symbiotic bacteria. Nat Rev Microbiol. 2012;10:13–26.
Reis F, Kirsch R, Pauchet Y, Bauer E, Bilz LC, Fukumori K, et al. Bacterial symbionts support larval sap feeding and adult folivory in (semi-)aquatic reed beetles. Nat Commun. 2020;11:2964.
Łukasik P, Newton JA, Sanders JG, Hu Y, Moreau CS, Kronauer DJC, et al. The structured diversity of specialized gut symbionts of the New World army ants. Mol Ecol. 2017;26:3808–25.
Arkin AP, Cottingham RW, Henry CS, Harris NL, Stevens RL, Maslov S, et al. KBase: the United States Department of Energy Systems Biology Knowledgebase. Nat Biotechnol. 2018;36:566–9.
Bolger AM, Lohse M, Usadel B. Trimmomatic: a flexible trimmer for Illumina sequence data. Bioinformatics. 2014;30:2114–20.
Li D, Luo R, Liu C-M, Leung C-M, Ting H-F, Sadakane K, et al. MEGAHIT v1.0: a fast and scalable metagenome assembler driven by advanced methodologies and community practices. Methods. 2016;102:3–11.
Kang DD, Li F, Kirton E, Thomas A, Egan R, An H, et al. MetaBAT 2: an adaptive binning algorithm for robust and efficient genome reconstruction from metagenome assemblies. PeerJ. 2019;7:e7359.
Wu Y-W, Simmons BA, Singer SW. MaxBin 2.0: an automated binning algorithm to recover genomes from multiple metagenomic datasets. Bioinformatics. 2016;32:605–7.
Sieber CMK, Probst AJ, Sharrar A, Thomas BC, Hess M, Tringe SG, et al. Recovery of genomes from metagenomes via a dereplication, aggregation and scoring strategy. Nat Microbiol. 2018;3:836–43.
Parks DH, Imelfort M, Skennerton CT, Hugenholtz P, Tyson GW. CheckM: assessing the quality of microbial genomes recovered from isolates, single cells, and metagenomes. Genome Res. 2015;25:1043–55.
Aziz RK, Bartels D, Best AA, DeJongh M, Disz T, Edwards RA, et al. The RAST server: rapid annotations using subsystems technology. BMC Genom. 2008;9:75.
Beukes CW, Palmer M, Manyaka P. Genome data provides high support for generic boundaries in Burkholderia sensu lato. Front Microbiol. 2017;8:1154.
Price MN, Dehal PS, Arkin AP. FastTree 2 – approximately maximum-likelihood trees for large alignments. PLoS ONE. 2010;5:e9490.
Tamura K, Stecher G, Kumar S. MEGA11: molecular evolutionary genetics analysis version 11. Mol Biol Evol. 2021;38:3022–7.
Gu Z, Eils R, Schlesner M. Complex heatmaps reveal patterns and correlations in multidimensional genomic data. Bioinformatics. 2016;32:2847–9.
Gilchrist CLM, Chooi Y-H. Clinker & clustermap.js: automatic generation of gene cluster comparison figures. Bioinformatics. 2021;37:2473–5.
Marçais G, Delcher AL, Phillippy AM, Coston R, Salzberg SL, Zimin A. MUMmer4: a fast and versatile genome alignment system. PLoS Comput Biol. 2018;14:e1005944.
McCutcheon J, McDonald B, Moran N. Convergent evolution of metabolic roles in bacterial co-symbionts of insects. Proc Natl Acad Sci USA. 2009;106:15394–9.
Santos-Garcia D, Latorre A, Moya A, Gibbs G, Hartung V, Dettner K, et al. Small but powerful, the primary endosymbiont of moss bugs, Candidatus Evansia muelleri, holds a reduced genome with large biosynthetic capabilities. Genome Biol Evol. 2014;6:1875–93.
Boucias DG, Garcia-Maruniak A, Cherry R, Lu H, Maruniak JE, Lietze V-U. Detection and characterization of bacterial symbionts in the heteropteran, Blissus insularis. FEMS Microbiol Ecol. 2012;82:629–41.
Ohbayashi T, Futahashi R, Terashima M, Barrière Q, Lamouche F, Takeshita K, et al. Comparative cytology, physiology and transcriptomics of Burkholderia insecticola in symbiosis with the bean bug Riptortus pedestris and in culture. ISME J. 2019;13:1469–83.
Kikuchi Y, Hayatsu M, Hosokawa T, Nagayama A, Tago K, Fukatsu T. Symbiont-mediated insecticide resistance. Proc Natl Acad Sci USA. 2012;109:8618–22.
Ishii Y, Matsuura Y, Kakizawa S, Nikoh N, Fukatsu T. Diversity of bacterial endosymbionts associated with Macrosteles leafhoppers vectoring phytopathogenic phytoplasmas. Appl Environ Microbiol. 2013;79:5013–22.
Szabó G, Schulz F, Manzano-Marín A, Toenshoff ER, Horn M. Evolutionarily recent dual obligatory symbiosis among adelgids indicates a transition between fungus- and insect-associated lifestyles. ISME J. 2022;16:247–56.
Partida-Martinez LP, Hertweck C. Pathogenic fungus harbours endosymbiotic bacteria for toxin production. Nature. 2005;437:884–8.
Estrada-de los Santos P, Palmer M, Chávez-Ramírez B, Beukes C, Steenkamp ET, Briscoe L, et al. Whole genome analyses suggests that Burkholderia sensu lato contains two additional novel genera (Mycetohabitans gen. nov., and Trinickia gen. nov.): implications for the evolution of diazotrophy and nodulation in the Burkholderiaceae. Genes. 2018;9:389.
Haselkorn TS, DiSalvo S, Miller JW, Bashir U, Brock DA, Queller DC, et al. The specificity of Burkholderia symbionts in the social amoeba farming symbiosis: Prevalence, species, genetic and phenotypic diversity. Mol Ecol. 2019;28:847–62.
Brock DA, Noh S, Hubert ANM, Haselkorn TS, DiSalvo S, Suess MK, et al. Endosymbiotic adaptations in three new bacterial species associated with Dictyostelium discoideum: Paraburkholderia agricolaris sp. nov., Paraburkholderia hayleyella sp. nov., and Paraburkholderia bonniea sp. nov. PeerJ. 2020;8:e9151.
Khojandi N, Haselkorn TS, Eschbach MN, Naser RA, DiSalvo S. Intracellular Burkholderia symbionts induce extracellular secondary infections; driving diverse host outcomes that vary by genotype and environment. ISME J. 2019;13:2068–81.
Dale C, Plague GR, Wang B, Ochman H, Moran NA. Type III secretion systems and the evolution of mutualistic endosymbiosis. Proc Natl Acad Sci USA. 2002;99:12397.
Maire J, Chouaia B, Zaidman-Rémy A, Heddi A. Endosymbiosis morphological reorganization during metamorphosis diverges in weevils. Commun Integr Biol. 2020;13:184–8.
Moebius N, Üzüm Z, Dijksterhuis J, Lackner G, Hertweck C. Active invasion of bacteria into living fungal cells. eLife 2014;3:e03007.
Douglas AE. Insects and their beneficial microbes. Princeton University Press; Princeton, Oxford. 2022.
Wu D, Daugherty SC, Van Aken SE, Pai GH, Watkins KL, Khouri H, et al. Metabolic complementarity and genomics of the dual bacterial symbiosis of sharpshooters. PLoS Biol. 2006;4:e188.
Łukasik P, Nazario K, Van Leuven JT, Campbell MA, Meyer M, Michalik A, et al. Multiple origins of interdependent endosymbiotic complexes in a genus of cicadas. Proc Natl Acad Sci USA. 2018;115:E226.
Michalik A, Castillo Franco D, Kobiałka M, Szklarzewicz T, Stroiński A, Łukasik P. Alternative transmission patterns in independently acquired nutritional co-symbionts of Dictyopharidae planthoppers. mBio. 2021;12:e01228–21.
Zhang Y, Rodionov DA, Gelfand MS, Gladyshev VN. Comparative genomic analyses of nickel, cobalt and vitamin B12 utilization. BMC Genom. 2009;10:78.
Hansen A, Moran N. Aphid genome expression reveals host–symbiont cooperation in the production of amino acids. PNAS. 2011;108:2849–54.
Husnik F, Nikoh N, Koga R, Ross L, Duncan RP, Fujie M, et al. Horizontal gene transfer from diverse bacteria to an insect genome enables a tripartite nested mealybug symbiosis. Cell. 2013;153:1567–78.
Bennett GM, Mao M. Comparative genomics of a quadripartite symbiosis in a planthopper host reveals the origins and rearranged nutritional responsibilities of anciently diverged bacterial lineages. Environ Microbiol. 2018;20:4461–72.
Tamas I, Wernegreen JJ, Nystedt B, Kauppinen SN, Darby AC, Gomez-Valero L, et al. Endosymbiont gene functions impaired and rescued by polymerase infidelity at poly(A) tracts. Proc Natl Acad Sci USA. 2008;105:14934–9.
Manzano-Marín A, Coeur d’acier A, Clamens A-L, Cruaud C, Barbe V, Jousselin E. Co-obligate symbioses have repeatedly evolved across aphids, but partner identity and nutritional contributions vary across lineages. Peer Community J. 2023;3:e46.
Husnik F, McCutcheon JP. Repeated replacement of an intrabacterial symbiont in the tripartite nested mealybug symbiosis. Proc Natl Acad Sci USA. 2016;113:E5416.
Tamas I, Klasson L, Canbäck B, Näslund AK, Eriksson A-S, Wernegreen JJ, et al. 50 million years of genomic stasis in endosymbiotic bacteria. Science. 2002;296:2376–9.
Mao M, Yang X, Bennett GM. Evolution of host support for two ancient bacterial symbionts with differentially degraded genomes in a leafhopper host. Proc Natl Acad Sci USA. 2018;115:E11691.
Michalik A, Castillo Franco D, Deng J, Szklarzewicz T, Stroiński A, Kobiałka M, et al. Variable organization of symbiont-containing tissue across planthoppers hosting different heritable endosymbionts. Front Physiol. 2023;14:1135346.
Toju H, Tanabe AS, Notsu Y, Sota T, Fukatsu T. Diversification of endosymbiosis: replacements, co-speciation and promiscuity of bacteriocyte symbionts in weevils. ISME J. 2013;7:1378–90.
Garber AI, Kupper M, Laetsch DR, Weldon SR, Ladinsky MS, Bjorkman PJ, et al. The evolution of interdependence in a four-way mealybug symbiosis. Genome Biol Evol. 2021;13:evab123.
Michalik A, Jankowska W, Szklarzewicz T. Ultrastructure and transovarial transmission of endosymbiotic microorganisms in Conomelus anceps and Metcalfa pruinosa (Insecta, Hemiptera, Fulgoromorpha). Folia Biol Kraków. 2009;57:131–7.
Kobiałka M, Michalik A, Szwedo J, Szklarzewicz T. Diversity of symbiotic microbiota in Deltocephalinae leafhoppers (Insecta, Hemiptera, Cicadellidae). Arthropod Struct Dev. 2018;47:268–78.
Koga R, Bennett GM, Cryan JR, Moran NA. Evolutionary replacement of obligate symbionts in an ancient and diverse insect lineage. Environ Microbiol. 2013;15:2073–81.
Bennett GM, Moran NA. Heritable symbiosis: the advantages and perils of an evolutionary rabbit hole. Proc Natl Acad Sci USA. 2015;112:10169.
Chong RA, Moran NA. Evolutionary loss and replacement of Buchnera, the obligate endosymbiont of aphids. ISME J. 2018;12:898–908.
Mao M, Bennett GM. Symbiont replacements reset the co-evolutionary relationship between insects and their heritable bacteria. ISME J. 2020;14:1384–95.
McCutcheon J, Boyd B, Dale C. The life of an insect endosymbiont from the cradle to the grave. Curr Biol. 2019;29:R485–R495.
Arrese EL, Soulages JL. Insect fat body: energy, metabolism, and regulation. Annu Rev Entomol. 2010;55:207–25.
Funding
This project was supported by the Polish National Science Centre grant 2021/41/B/NZ8/04526 (to AM) and the ERC Consolidator Grant ‘SYMBeetle’ (ERC CoG 819585 to MK). Open Access funding enabled and organized by Projekt DEAL.
Author information
Authors and Affiliations
Contributions
AM, EB, and MK conceived the study. AM and TSz obtained samples and performed the microscopy, EB carried out the genome assembly and annotation. EB, AM, and MK analyzed the data. AM, EB, TSz, and MK wrote the paper.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Michalik, A., Bauer, E., Szklarzewicz, T. et al. Nutrient supplementation by genome-eroded Burkholderia symbionts of scale insects. ISME J 17, 2221–2231 (2023). https://doi.org/10.1038/s41396-023-01528-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41396-023-01528-4