Abstract
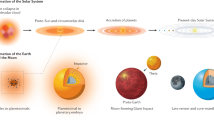
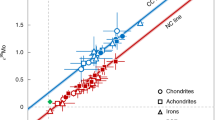
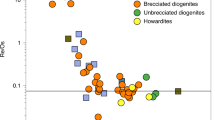
Kelvin calculated the age of the Earth to be about 24 million years by assuming conductive cooling from being fully molten to its current state1. Although simplistic2, his result is interesting in the context of the dramatic cooling that took place after the putative Moon-forming giant impact, which contributed the final ∼10 per cent of the Earth's mass3,4. The rate of accretion and core segregation on Earth as deduced from the U–Pb system5 is much slower than that obtained from Hf–W systematics6,7,8, and implies substantial accretion after the Moon-forming impact, which occurred 45 ± 5 Myr after the beginning of the Solar System. Here we propose an explanation for the two timescales5,9. We suggest that the Hf–W timescale reflects the principal phase of core-formation before the giant impact. Crystallization of silicate perovskite in the lower mantle during this phase produced Fe3+, which was released during the giant impact10, and this oxidation resulted in late segregation of sulphur-rich metal into which Pb dissolved readily, setting the younger U–Pb age of the Earth. Separation of the latter metal then occurred 30 ± 10 Myr after the Moon-forming impact. Over this time span, in surprising agreement with Kelvin's result, the Earth cooled by about 4,000 K in returning from a fully molten to a partially crystalline state.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Kelvin, The age of the Earth. Nature 51, 438–440 (1895)
Carslaw, H. S. & Jaeger, J. C. Conduction of Heat in Solids (Oxford Univ. Press, London, 1959)
Cameron, A. G. W. & Benz, W. Origin of the Moon and the single impact hypothesis IV. Icarus 92, 204–216 (1991)
Canup, R. M. & Asphaug, E. Origin of the Moon in a giant impact near the end of the Earth's formation. Nature 412, 708–712 (2001)
Halliday, A. N. Mixing, volatile loss and compositional change during impact-driven accretion of the Earth. Nature 427, 505–509 (2004)
Kleine, T., Munker, C., Mezger, K. & Palme, H. Rapid accretion and early core formation on asteroids and the terrestrial planets from Hf-W chronometry. Nature 418, 952–955 (2002)
Yin, Q. Z. et al. A short timescale for terrestrial planet formation from Hf-W chronometry of meteorites. Nature 418, 949–952 (2002)
Schoenberg, R., Kamber, B. S., Collerson, K. D. & Eugster, O. New W-isotope evidence for rapid terrestrial accretion and very early core formation. Geochim. Cosmochim. Acta 66, 3151–3160 (2002)
Kleine, T., Palme, H. & Mezger, K. The Hf-W age of the lunar magma ocean. Proc. Lunar Planet. Sci. Conf. XXXVI, 1940 (2005)
Frost, D. J. et al. Experimental evidence for the existence of iron-rich metal in the Earth's lower mantle. Nature 428, 409–412 (2004)
Halliday, A. N. The origin and earliest history of the Earth. In Meteorites, Comets and Planets (ed. Davis, A. M.) 509–557 (Elsevier-Pergamon, Oxford, 2003)
Stevenson, D. J. Models of the Earth's core. Science 214, 611–619 (1981)
Harper, C. L. Jr & Jacobsen, S. B. Evidence for 182Hf in the early Solar System and constraints on the timescale of terrestrial accretion and core formation. Geochim. Cosmochim. Acta 60, 1131–1153 (1996)
Canup, R. M. Simulations of a late lunar-forming impact. Icarus 168, 433–456 (2004)
Halliday, A. N. & Kleine, T. Meteorites and the timing, mechanisms and conditions of terrestrial planet accretion and early differentiation. In Meteorites and the Early Solar System II (eds Lauretta, D., Leshin, L. & MacSween, H.) (Univ. Arizona Press, Tucson, in the press)
McDonough, W. F. & Sun, S.-s. The composition of the Earth. Chem. Geol. 120, 223–253 (1995)
Allegre, C. J., Poirier, J.-P., Humler, E. & Hofmann, A. W. The chemical composition of the Earth. Earth Planet. Sci. Lett. 134, 515–526 (1995)
Righter, K. & Drake, M. J. Metal-silicate equilibrium in a homogeneously accreting earth: New results for Re. Earth Planet. Sci. Lett. 146, 541–553 (1997)
Ohtani, E., Yurimoto, H. & Seto, S. Element partitioning between metallic liquid, silicate liquid, and lower-mantle minerals: implications for core formation of the Earth. Phys. Earth Planet. Inter. 100, 97–114 (1997)
Wood, B. J., Bryndzia, L. T. & Johnson, K. E. Mantle oxidation state and its relationship to tectonic environment and fluid speciation. Science 248, 337–345 (1990)
Francis, R. D. Sulfide globules in mid-ocean ridge basalts (MORB), and the effect of oxygen abundance in Fe-S-O liquids on the ability of those liquids to partition metals from MORB and komatiite magmas. Chem. Geol. 85, 199–213 (1990)
Jones, J. H., Hart, S. R. & Benjamin, T. M. Experimental partitioning near the Fe-FeS eutectic, with an emphasis on elements important to iron meteorite chronologies (Pb, Ag, Pd, and Tl). Geochim. Cosmochim. Acta 57, 453–460 (1993)
Chabot, N. L. & Jones, J. H. The parameterization of solid metal-liquid metal partitioning of siderophile elements. Meteorit. Planet. Sci. 38, 1425–1436 (2003)
O'Neill, H. S. The origin of the Moon and the early history of the Earth—A chemical model. Part 2: The Earth. Geochim. Cosmochim. Acta 55, 1159–1172 (1991)
Kasting, J. F., Eggler, D. H. & Raeburn, S. P. Mantle redox evolution and the oxidation-state of the Archean atmosphere. J. Geol. 101, 245–257 (1993)
Chabot, N. L., Draper, D. S. & Agee, C. B. Conditions of core formation in the Earth: Constraints from nickel and cobalt partitioning. Geochim. Cosmochim. Acta 69, 2141–2151 (2005)
Wade, J. & Wood, B. J. Core formation and the oxidation state of the Earth. Earth Planet. Sci. Lett. 236, 78–95 (2005)
Yi, W. et al. Cadmium, indium, tin, tellurium, and sulfur in oceanic basalts: Implications for chalcophile element fractionation in the Earth. J. Geophys. Res. Solid Earth 105, 18927–18948 (2000)
Holzheid, A. & Grove, T. L. Sulfur saturation limits in silicate melts and their implications for core formation scenarios for terrestrial planets. Am. Mineral. 87, 227–237 (2002)
Abe, Y. Thermal and chemical evolution of the terrestrial magma ocean. Phys. Earth Planet. Inter. 100, 27–39 (1997)
Wetherill, G. W. Accumulation of the terrestrial planets and implications concerning lunar origin. In Origin of the Moon (eds Hartmann, W. K., Phillips, R. J. & Taylor, G. J.) (Lunar and Planetary Institute, Houston, 1986)
Tronnes, R. G. & Frost, D. J. Peridotite melting and mineral-melt partitioning of major and minor elements at 22–24.5 GPa. Earth Planet. Sci. Lett. 197, 117–131 (2002)
Kilinc, A., Carmichael, I. S. E., Rivers, M. L. & Sack, R. O. The ferric-ferrous ratio of natural silicate liquids equilibrated in air. Contrib. Mineral. Petrol. 83, 136–140 (1983)
Acknowledgements
Discussions with J. Wade and M. Walter helped to clarify our arguments. B.J.W. acknowledges the support of a Max-Planck Research Award.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
Reprints and permissions information is available at npg.nature.com/reprintsandpermissions. The authors declare no competing financial interests.
Rights and permissions
About this article
Cite this article
Wood, B., Halliday, A. Cooling of the Earth and core formation after the giant impact. Nature 437, 1345–1348 (2005). https://doi.org/10.1038/nature04129
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1038/nature04129
This article is cited by
-
Magma Ocean, Water, and the Early Atmosphere of Venus
Space Science Reviews (2023)
-
The accretion of planet Earth
Nature Reviews Earth & Environment (2022)
-
Stratification in planetary cores by liquid immiscibility in Fe-S-H
Nature Communications (2022)
-
An upper limit on late accretion and water delivery in the TRAPPIST-1 exoplanet system
Nature Astronomy (2021)
-
Formation of Venus, Earth and Mars: Constrained by Isotopes
Space Science Reviews (2021)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.