Abstract
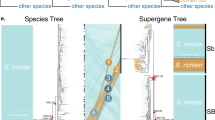
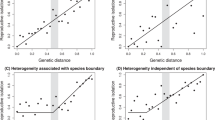
The reinforcement model of evolution argues that natural selection enhances pre-zygotic isolation between divergent populations or species by selecting against unfit hybrids1,2 or costly interspecific matings3. Reinforcement is distinguished from other models that consider the formation of reproductive isolation to be a by-product of divergent evolution4,5. Although theory has shown that reinforcement is a possible mechanism that can lead to speciation6,7,8, empirical evidence has been sufficiently scarce to raise doubts about the importance of reinforcement in nature6,9,10. Agrodiaetus butterflies (Lepidoptera: Lycaenidae) exhibit unusual variability in chromosome number. Whereas their genitalia and other morphological characteristics are largely uniform, different species vary considerably in male wing colour, and provide a model system to study the role of reinforcement in speciation. Using comparative phylogenetic methods, we show that the sympatric distribution of 15 relatively young sister taxa of Agrodiaetus strongly correlates with differences in male wing colour, and that this pattern is most likely the result of reinforcement. We find little evidence supporting sympatric speciation: rather, in Agrodiaetus, karyotypic changes accumulate gradually in allopatry, prompting reinforcement when karyotypically divergent races come into contact.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Dobzhansky, T. Speciation as a stage in evolutionary divergence. Am. Nat. 74, 312–321 (1940)
Noor, M. A. F. Speciation driven by natural selection in Drosophila. Nature 375, 674–675 (1995)
Coyne, J. A. & Orr, A. H. Speciation (Sinauer Associates, Sunderland, Massachusetts, 2004)
Mayr, E. Population, Species, and Evolution; an Abridgment of Animal Species and Evolution (Harvard Univ. Press, Cambridge, Massachusetts, 1970)
Turelli, M., Barton, N. & Coyne, J. Theory and speciation. Trends Ecol. Evol. 16, 330–343 (2001)
Noor, M. A. F. Reinforcement and other consequences of sympatry. Heredity 83, 503–508 (1999)
Kirkpatrick, M. & Ravigne, V. Speciation by natural and sexual selection: models and experiments. Am. Nat. 159, S22–S35 (2002)
Servedio, M. R. & Noor, M. A. F. The role of reinforcement in speciation: theory and data. Annu. Rev. Ecol. Evol. Syst. 34, 339–364 (2003)
Butlin, R. K. Species, speciation, and reinforcement. Am. Nat. 130, 461–464 (1987)
Marshall, J. L., Arnold, M. L. & Howard, D. J. Reinforcement: the road not taken. Trends Ecol. Evol. 17, 558–563 (2002)
Coyne, J. A. & Orr, H. A. Patterns of speciation in Drosophila. Evolution 43, 362–381 (1989)
Coyne, J. A. & Orr, A. H. “Patterns of speciation in Drosophila” revisited. Evolution 51, 295–303 (1997)
Sætre, G.-P. et al. A sexually selected character displacement in flycatchers reinforces premating isolation. Nature 387, 589–592 (1997)
Jiggins, C. D., Naisbit, R. E., Coe, R. L. & Mallet, J. Reproductive isolation caused by colour pattern mimicry. Nature 411, 302–305 (2001)
Nosil, P., Crespi, B. J. & Sandoval, C. P. Reproductive isolation driven by combined effects of ecological adaptation and reinforcement. Proc. R. Soc. Lond. B 270, 1911–1918 (2003)
Templeton, A. R. Mechanisms of speciation – a population genetic approach. Annu. Rev. Ecol. Syst. 12, 23–48 (1981)
Butlin, R. K. Reinforcement: an idea evolving. Trends Ecol. Evol. 10, 432–434 (1995)
Day, T. Sexual selection and the evolution of costly female preference: spatial effects. Evolution 54, 715–730 (2000)
Kandul, N. P. et al. Phylogeny of Agrodiaetus Hübner 1822 (Lepidoptera: Lycaenidae) inferred from mtDNA sequences of COI and COII, and nuclear sequences of EF1-a: karyotype diversification and species radiation. Syst. Biol. 53, 278–298 (2004)
Lesse, de H. Spéciation et variation chromosomique chez les Lépidoptères Rhopalocères. Ann. Sci. Nat. Zool. Biol. Anim. 2, 1–223 (1960)
Lukhtanov, V. A. & Danchenko, A. D. Principles of the highly ordered arrangement of metaphase I bivalents in spermatocytes of Agrodiaetus (Insecta, Lepidoptera). Chromosome Res. 10, 5–20 (2002)
Hagen, W. T. Freilandhybriden bei Blaeulingen aus Ostanatolien und Iran (Lepidoptera: Lycaenidae). Nachr. entomol. Ver. Apollo 23, 199–203 (2003)
Schurian, K. G. & Hofmann, P. Ein neuer Lycaeniden-Hybrid: Agrodiaetus ripartii Freyer x Agrodiaetus menalcas Freyer (Lepidoptera: Lycaenidae). Nachr. entomol. Ver. Apollo 1, 21–23 (1980)
Lorkovíc, Z. in Butterflies of Europe (ed. Kudrna, O.) 332–396 (Aula, Wiesbaden, 1990)
Fordyce, J. A., Nice, C. C., Forister, M. L. & Shapiro, A. M. The significance of wing pattern diversity in the Lycaenidae: mate discrimination by two recently diverged species. J. Evol. Biol. 14, 871–879 (2002)
Vane-Wright, R. I. & Boppre, M. Visual and chemical signalling in butterflies: functional and phylogenetic perspective. Phil. Trans. R. Soc. Lond. B 340, 197–205 (1993)
Bernard, G. D. & Remington, C. Color vision in Lycaena butterflies: spectral tuning of receptor arrays in relation to behavioural ecology. Proc. Natl Acad. Sci. USA 88, 2783–2787 (1991)
Drummond, B. A. in Sperm Competition and the Evolution of Animal Mating Systems (ed. Smith, R. L.) 291–370 (Academic, San Diego, California, 1984)
Felsenstein, J. Phylogenies and the comparative method. Am. Nat. 125, 1–15 (1985)
Barraclough, T. G. & Vogler, A. P. Detecting the geographic pattern of speciation from species-level phylogeny. Am. Nat. 155, 419–434 (2000)
Acknowledgements
We thank A. J. Berry, J. A. Coyne, S. V. Edwards, J. R. Morris and R. Vila for their advice during the preparation of this manuscript. C. Bilgin, J. Coleman, F. Fernández-Rubio, G. Grigorjev, J. Jubany, C. Ibánez, R. Martínez, M. L. Munguira, C. Sekercioglu, C. Stefanescu, M. A. Travassos, R. Vila and V. Zurilina helped with collecting specimens, and J. Coleman and R. Vila assisted with sequencing in the laboratory. W. H. Piel and A. Monteiro helped us to measure wing ultraviolet reflectance. This research was supported by three collecting grants from the Putnam Expeditionary Fund of the Museum of Comparative Zoology, Harvard University; a National Science Foundation Doctoral Dissertation Improvement Grant to N.P.K.; National Science Foundation and Baker Foundation grants to N.E.P.; Milton Fund grants to D.H. and J.B.P.; a Burroughs Wellcome Fund grant to J.B.P.; and grants from the Russian Foundation for Basic Research, and the Russian Federal Programs “Universities of Russia” and “Leading Scientific Schools” to V.A.L.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The sequences have been deposited in GenBank; see Supplementary Appendix 1 for details. Reprints and permissions information is available at npg.nature.com/reprintsandpermissions. The authors declare no competing financial interests.
Supplementary information
Supplementary Notes
This file contains Supplementary Methods, Supplementary Figures S1-S7, Supplementary Tables S1-S5, Supplementary Discussion and additional references. (PDF 4253 kb)
Rights and permissions
About this article
Cite this article
Lukhtanov, V., Kandul, N., Plotkin, J. et al. Reinforcement of pre-zygotic isolation and karyotype evolution in Agrodiaetus butterflies. Nature 436, 385–389 (2005). https://doi.org/10.1038/nature03704
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1038/nature03704
This article is cited by
-
Chromosomal conservatism vs chromosomal megaevolution: enigma of karyotypic evolution in Lepidoptera
Chromosome Research (2023)
-
The slow-evolving Acorus tatarinowii genome sheds light on ancestral monocot evolution
Nature Plants (2022)
-
Highly divergent karyotypes and barcoding of the East African genus Gonatoxia Karsch (Orthoptera: Phaneropterinae)
Scientific Reports (2021)
-
Concordance of the spectral properties of dorsal wing scales with the phylogeographic structure of European male Polyommatus icarus butterflies
Scientific Reports (2021)
-
A morphometric analysis of environmental dependences between ultraviolet patches and wing venation patterns in Gonepteryx butterflies (Lepidoptera, Pieridae)
Evolutionary Ecology (2019)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.