Abstract
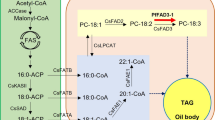
Efficient storage of carbon in seeds is crucial to plant fitness and to agricultural productivity. Oil is a major reserve material in most seeds1, and these oils provide the largest source of renewable reduced carbon chains available from nature. However, the conversion of carbohydrate to oil through glycolysis results in the loss of one-third of the carbon as CO2. Here we show that, in developing embryos of Brassica napus L. (oilseed rape), Rubisco (ribulose 1,5-bisphosphate carboxylase/oxygenase) acts without the Calvin cycle2 and in a previously undescribed metabolic context to increase the efficiency of carbon use during the formation of oil. In comparison with glycolysis, the metabolic conversion we describe provides 20% more acetyl-CoA for fatty-acid synthesis and results in 40% less loss of carbon as CO2. Our conclusions are based on measurements of mass balance, enzyme activity and stable isotope labelling, as well as an analysis of elementary flux modes.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
References
Levin, D. A. The oil content of seeds: An ecological perspective. Am. Nat. 108, 193–206 (1974)
Bassham, J. A. et al. The path of carbon in photosynthesis. XXI. The cyclic regeneration of carbon dioxide acceptor. J. Am. Chem. Soc. 67, 1760–1770 (1954)
Schwender, J., Ohlrogge, J. & Shachar-Hill, Y. Understanding flux in plant metabolic networks. Curr. Opin. Plant Biol. 7, 309–317 (2004)
Neuhaus, H. E. & Emes, M. J. Non-photosynthetic metabolism in plastids. Annu. Rev. Plant Physiol. Plant Mol. Biol. 51, 111–140 (2000)
King, S. P., Badger, M. R. & Furbank, R. T. CO2 refixation characteristics of developing canola seeds and silique wall. Aust. J. Plant Phys. 25, 377–386 (1998)
Schwender, J., Ohlrogge, J. B. & Shachar-Hill, Y. A flux model of glycolysis and the oxidative pentose phosphate pathway in developing Brassica napus embryos. J. Biol. Chem. 278, 29442–29453 (2003)
Ruuska, S. A., Schwender, J. & Ohlrogge, J. B. The capacity of green oilseeds to utilize photosynthesis to drive biosynthetic processes. Plant Physiol. 136, 2700–2709 (2004)
Goffman, F. D., Ruckle, M., Ohlrogge, J. B. & Shachar-Hill, Y. Carbon dioxide concentrations are very high in developing oil seeds. Plant Physiol. Biochem. 42, 703–708 (2004)
Schwender, J. & Ohlrogge, J. Probing in vivo metabolism by stable isotope labeling of storage lipids and proteins in developing Brassica napus embryos. Plant Physiol. 130, 347–361 (2002)
Schuster, S., Fell, D. & Dandekar, T. A general definition of metabolic pathways useful for systematic organization and analysis of complex metabolic networks. Nature Biotechnol. 18, 326–332 (2000)
Schuster, S., Dandekar, T. & Fell, D. A. Detection of elementary flux modes in biochemical networks: a promising tool for pathway analysis and metabolic engineering. Trends Biotechnol. 17, 53–60 (1999)
Acknowledgements
This work was supported by grants from the Department of Energy, the National Science Foundation and the USDA. Acknowledgement is also made to the Michigan Agricultural Experiment Station for its support of this research.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare that they have no competing financial interests.
Supplementary information
Supplementary Notes
Contains an analysis of how much carbon dioxide can be assimilated and stored in biomass by carboxylation reactions that produce oxaloacetate. Alternative metabolic routes for converting hexose into acetyl-CoA are described and analysed as elementary flux modes. The labelling experiments using 13CO2 or 13C-labelled alanine are discussed with additional detail. (DOC 354 kb)
Rights and permissions
About this article
Cite this article
Schwender, J., Goffman, F., Ohlrogge, J. et al. Rubisco without the Calvin cycle improves the carbon efficiency of developing green seeds. Nature 432, 779–782 (2004). https://doi.org/10.1038/nature03145
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1038/nature03145
This article is cited by
-
The light and hypoxia induced gene ZmPORB1 determines tocopherol content in the maize kernel
Science China Life Sciences (2024)
-
Evaluation of oil accumulation and biodiesel property of Lindera glauca fruits among different germplasms and revelation of high oil producing mechanism for developing biodiesel
Biotechnology for Biofuels and Bioproducts (2023)
-
Improving fatty acid composition of soybean yield under NaCl stress by soaking seeds in ascorbate
Acta Physiologiae Plantarum (2023)
-
High oil accumulation in tuber of yellow nutsedge compared to purple nutsedge is associated with more abundant expression of genes involved in fatty acid synthesis and triacylglycerol storage
Biotechnology for Biofuels (2021)
-
Analysis of fatty acid compositions and differential gene expression in two Iranian olive cultivars during fruit ripening
Acta Physiologiae Plantarum (2021)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.