Abstract
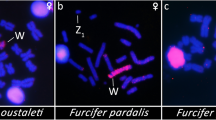
Two centuries after the duck-billed platypus was discovered, monotreme chromosome systems remain deeply puzzling. Karyotypes of males1, or of both sexes2,3,4, were claimed to contain several unpaired chromosomes (including the X chromosome) that form a multi-chromosomal chain at meiosis. Such meiotic chains exist in plants5 and insects6 but are rare in vertebrates7. How the platypus chromosome system works to determine sex and produce balanced gametes has been controversial for decades1,2,3,4. Here we demonstrate that platypus have five male-specific chromosomes (Y chromosomes) and five chromosomes present in one copy in males and two copies in females (X chromosomes). These ten chromosomes form a multivalent chain at male meiosis, adopting an alternating pattern to segregate into XXXXX-bearing and YYYYY-bearing sperm. Which, if any, of these sex chromosomes bears one or more sex-determining genes remains unknown. The largest X chromosome, with homology to the human X chromosome, lies at one end of the chain, and a chromosome with homology to the bird Z chromosome lies near the other end. This suggests an evolutionary link between mammal and bird sex chromosome systems, which were previously thought to have evolved independently.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Bick, Y. in The Meiotic Chain of Chromosomes of Monotremata (ed. Augee, M.) 64–68 (Royal Zoological Society of New South Wales, Sydney, 1992)
Murtagh, C. A unique cytogenetics system in monotremes. Chromosoma 65, 37–57 (1977)
Bick, Y. & Sharman, G. The chromosomes of the platypus (Ornithorhynchus): Monotremata. Cytobios 14, 17–28 (1975)
Wrigley, J. M. & Graves, J. A. M. Karyotypic conservation in the mammalian order Monotremata (subclass Prototheria). Chromosoma 96, 231–247 (1988)
Cleland, R. The Cytogenetics of Oenothera (Academic, New York, 1962)
Syren, R. M. & Luykx, P. Permanent segmental interchange complex in the termite Incisitermes schwarzi. Nature 266, 167–168 (1977)
Fredga, K. Unusual sex chromosome inheritance in mammals. Phil. Trans R. Soc. Lond. B 259, 15–36 (1970)
Woodburne, M. O., Rich, T. H. & Springer, M. S. The evolution of tribospheny and the antiquity of mammalian clades. Mol. Phylogenet. Evol. 28, 360–385 (2003)
Grützner, F., Deakin, J., Rens, W., El-Mogharbel, N. & Marshall Graves, J. A. M. The monotreme genome: a patchwork of reptile, mammal and unique features? Comp. Biochem. Physiol. A Mol. Integr. Physiol. 136, 867–881 (2003)
Graves, J. A. M. The origin and function of the mammalian Y chromosome and Y-borne genes—an evolving understanding. Bioessays 17, 311–320 (1995)
Griffiths, R. The isolation of conserved DNA sequences related to the human sex-determining region Y gene from the lesser black-backed gull (Larus fuscus). Proc. R. Soc. Lond. B 244, 123–128 (1991)
Mizuno, S. et al. Z and W chromosomes of chickens: studies on their gene functions in sex determination and sex differentiation. Cytogenet. Genome Res. 99, 236–244 (2002)
Nanda, I. et al. 300 million years of conserved synteny between chicken Z and human chromosome 9. Nature Genet. 21, 258–259 (1999)
Shetty, S., Kirby, P., Zarkower, D. & Graves, J. A. DMRT1 in a ratite bird: evidence for a role in sex determination and discovery of a putative regulatory element. Cytogenet. Genome Res. 99, 245–251 (2002)
Matsuda, M. et al. DMY is a Y-specific DM-domain gene required for male development in the medaka fish. Nature 417, 559–563 (2002)
Nanda, I. et al. A duplicated copy of DMRT1 in the sex-determining region of the Y chromosome of the medaka, Oryzias latipes. Proc. Natl Acad. Sci. USA 99, 11778–11783 (2002)
Raymond, C. S., Murphy, M. W., O'Sullivan, M. G., Bardwell, V. J. & Zarkower, D. Dmrt1, a gene related to worm and fly sexual regulators, is required for mammalian testis differentiation. Genes Dev. 14, 2587–2595 (2000)
Ohno, S. Chromosomes and Sex Linked Genes (Springer, Berlin, 1967)
Rens, W. et al. Resolution and evolution of the duck-billed platypus karyotype with a X1Y1X2Y2X3Y3X4Y4X5Y5 male sex chromosome constitution. Proc. Natl Acad. Sci. USA (in the press)
Johannisson, R. & Winking, H. Synaptonemal complexes of chains and rings in mice heterozygous for multiple Robertsonian translocations. Chromosome Res. 2, 137–145 (1994)
Eaker, S., Pyle, A., Cobb, J. & Handel, M. A. Evidence for meiotic spindle checkpoint from analysis of spermatocytes from Robertsonian-chromosome heterozygous mice. J. Cell Sci. 114, 2953–2965 (2001)
Toder, R. et al. Comparative chromosome painting between two marsupials: origins of an XX/XY1Y2 sex chromosome system. Mamm. Genome 8, 418–422 (1997)
Rahn, M. I., Mudry, M., Merani, M. S. & Solari, A. J. Meiotic behavior of the X1X2Y1Y2 quadrivalent of the primate Alouatta caraya. Chromosome Res. 4, 350–356 (1996)
Charlesworth, B. The evolution of sex chromosomes. Science 251, 1030–1033 (1991)
Watson, J. M., Meyne, J. & Graves, J. A. M. in Platypus and Echidnas (ed. Augee, M.) 53–63 (Royal Zoological Society of New South Wales, Sydney, 1992)
Rens, W., O'Brien, P. C., Yang, F., Graves, J. A. & Ferguson-Smith, M. A. Karyotype relationships between four distantly related marsupials revealed by reciprocal chromosome painting. Chromosome Res. 7, 461–474 (1999)
Telenius, H. et al. Degenerate oligonucleotide-primed PCR: general amplification of target DNA by a single degenerate primer. Genomics 13, 718–725 (1992)
Wrigley, J. M. & Graves, J. A. M. Two monotreme cell lines, derived from female platypuses (Ornithorhynchus anatinus; Monotremata, Mammalia). In Vitro 20, 321–328 (1984)
Brunner, B. et al. Genomic organization and expression of the doublesex-related gene cluster in vertebrates and detection of putative regulatory regions for DMRT1. Genomics 77, 8–17 (2001)
Acknowledgements
We thank P. Miethke and D. McMillan for technical assistance, D. Zarkower for providing the chicken DMRT1 cDNA and A. Pask for information on SOX genes and SRY in monotremes, and provision of Supplementary Fig. 1. This work was supported by the Australian Research Council (F.G. and J.A.M.G.). W.R. and M.A.F.-S. are supported by a Wellcome Trust grant to the Cambridge Resource Centre for Comparative Genomics. Facilities were provided by Macquarie Generation and Glenrock Station, NSW. Approval to collect animals was granted by the New South Wales National Parks and Wildlife Services, New South Wales Fisheries, Environment Australian Capital Territory and the Animal Experimentation Ethics Committee, Australian National University.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare that they have no competing financial interests.
Supplementary information
Supplementary Note
Search for Platypus SRY (DOC 31 kb)
Supplementary Figure 1
Male and female platypus DNA cut with EcoRI and probed with tammar wallaby SOX3. (PDF 2102 kb)
Supplementary Figure 2
DAPI inverted karyotype of male platypus taken from a male cell. (PDF 278 kb)
Supplementary Figure 3
Amino-acid sequence alignment of platypus DMRT1 with DMRT1 from different species. (PDF 106 kb)
Supplementary Table 1
Chromosome paints derived from elements of the translocation chain. (DOC 20 kb)
Supplementary Table 2
a, Alternate segregation of E-odd and E-even elements of the chain. b, Co-segregation of E-odd elements. (DOC 24 kb)
Rights and permissions
About this article
Cite this article
Grützner, F., Rens, W., Tsend-Ayush, E. et al. In the platypus a meiotic chain of ten sex chromosomes shares genes with the bird Z and mammal X chromosomes. Nature 432, 913–917 (2004). https://doi.org/10.1038/nature03021
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nature03021
This article is cited by
-
Findzx: an automated pipeline for detecting and visualising sex chromosomes using whole-genome sequencing data
BMC Genomics (2022)
-
Genetic sex test for the short-beaked echidna (Tachyglossus aculeatus)
Conservation Genetics Resources (2022)
-
Platypus and echidna genomes reveal mammalian biology and evolution
Nature (2021)
-
Do sex chromosomes of snakes, monitor lizards, and iguanian lizards result from multiple fission of an “ancestral amniote super-sex chromosome”?
Chromosome Research (2020)
-
Chromosome map of the Siamese cobra: did partial synteny of sex chromosomes in the amniote represent “a hypothetical ancestral super-sex chromosome” or random distribution?
BMC Genomics (2018)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.