Abstract
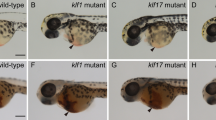
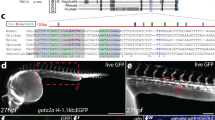
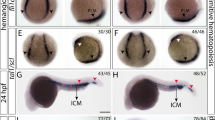
Organogenesis is dependent on the formation of distinct cell types within the embryo. Important to this process are the hox genes, which are believed to confer positional identities to cells along the anteroposterior axis1,2,3. Here, we have identified the caudal-related gene cdx4 as the locus mutated in kugelig (kgg), a zebrafish mutant with an early defect in haematopoiesis that is associated with abnormal anteroposterior patterning and aberrant hox gene expression. The blood deficiency in kgg embryos can be rescued by overexpressing hoxb7a or hoxa9a but not hoxb8a, indicating that the haematopoietic defect results from perturbations in specific hox genes. Furthermore, the haematopoietic defect in kgg mutants is not rescued by scl overexpression, suggesting that cdx4 and hox genes act to make the posterior mesoderm competent for blood development. Overexpression of cdx4 during zebrafish development or in mouse embryonic stem cells induces blood formation and alters hox gene expression. Taken together, these findings demonstrate that cdx4 regulates hox genes and is necessary for the specification of haematopoietic cell fate during vertebrate embryogenesis.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Lewis, E. A gene complex controlling segmentation in Drosophila. Nature 276, 565–570 (1978)
Struhl, G. Genes controlling segmental specification in Drosophila thorax. Proc. Natl Acad. Sci. USA 79, 7380–7384 (1982)
Hunt, P. & Krumlauf, R. Deciphering the Hox code: clues to patterning branchial regions of the head. Cell 66, 1075–1078 (1991)
Hammerschmidt, M. et al. Mutations affecting morphogenesis during gastrulation and tail formation in the zebrafish Danio rerio. Development 123, 143–151 (1996)
Mlodzik, M., Fjose, A. & Gehring, W. J. Isolation of caudal, a Drosophila homeo box-containing gene with maternal expression whose transcripts form a concentration gradient at the pre-blastoderm stage. EMBO J. 4, 2961–2969 (1985)
Katsuyama, Y., Sato, Y., Wada, S. & Saiga, H. Ascidian tail formation requires caudal function. Dev. Biol. 213, 257–268 (1999)
Edgar, L. G., Carr, S., Wang, H. & Wood, W. B. Zygotic expression of the caudal homolog pal-1 is required for posterior patterning in Caenorhabditis elegans embryogenesis. Dev. Biol. 229, 71–88 (2001)
Subramanian, V., Meyer, B. I. & Gruss, P. Disruption of the murine homeobox gene cdx1 affects axial skeletal identities by altering the mesodermal expression domains of Hox genes. Cell 83, 641–653 (1995)
Chawengsaksophak, K., James, R., Hammond, V. E., Kontgen, F. & Beck, F. Homeosis and intestinal tumours in cdx2 mutant mice. Nature 386, 84–87 (1997)
van den Akker, E. et al. Cdx1 and Cdx2 have overlapping functions in anteroposterior patterning and posterior axis elongation. Development 129, 2181–2193 (2002)
Beck, F., Chawengsaksophak, K., Waring, P., Playford, R. J. & Furness, J. B. Reprogramming of intestinal differentiation and intercalary regeneration in Cdx2 mutant mice. Proc. Natl Acad. Sci. USA 96, 7318–7323 (1999)
Tamai, Y. et al. Colonic hamartoma development by anomalous duplication in Cdx2 knockout mice. Cancer Res. 59, 2965–2970 (1999)
Charité, J. et al. Transducing positional information to the Hox genes: critical interaction of cdx gene products with position-sensitive regulatory elements. Development 125, 4349–4358 (1998)
Hunter, C. P., Harris, J. M., Maloof, J. N. & Kenyon, C. Hox gene expression in a single Caenorhabditis elegans cell is regulated by a caudal homolog and intercellular signals that inhibit Wnt signaling. Development 126, 805–814 (1999)
Owens, B. M. & Hawley, R. G. HOX and non-HOX homeobox genes in leukemic hematopoiesis. Stem Cells 20, 364–379 (2002)
Krumlauf, R. Hox genes in vertebrate development. Cell 78, 191–201 (1994)
Gering, M., Rodaway, A. R. F., Göttgens, B., Patient, R. K. & Green, A. R. The SCL gene specifies haemangioblast development from early mesoderm. EMBO J. 17, 4029–4045 (1998)
Sauvageau, G. et al. Overexpression of HOXB4 in hematopoietic cells causes the selective expansion of more primitive populations in vitro and in vivo. Genes Dev. 9, 1753–1765 (1995)
Antonchuk, J., Sauvageau, G. & Humphries, R. K. HOXB4-induced expansion of adult hematopoietic stem cells ex vivo. Cell 109, 39–45 (2002)
Buske, C. et al. Deregulated expression of HOXB4 enhances the primitive growth activity of human hematopoietic cells. Blood 100, 862–868 (2002)
Bjornsson, J. M. et al. Reduced proliferative capacity of hematopoietic stem cells deficient in hoxb3 and hoxb4. Mol. Cell. Biol. 23, 3872–3883 (2003)
Thorsteinsdottir, U. et al. Overexpression of the myeloid leukemia-associated Hoxa9 gene in bone marrow cells induces stem cell expansion. Blood 99, 121–129 (2002)
Perkins, A. C. & Cory, S. Conditional immortalization of mouse myelomonocytic, megakaryocytic and mast cell progenitors by the Hox-2.4 homeobox gene. EMBO J. 12, 3835–3846 (1993)
Chen, F., Greer, J. & Capecchi, M. R. Analysis of Hoxa7/Hoxb7 mutants suggests periodicity in the generation of the different sets of vertebrae. Mech. Dev. 77, 49–57 (1998)
Kappen, C. Disruption of the homeobox gene Hoxb-6 in mice results in increased numbers of early erythrocyte progenitors. Am. J. Hematol. 65, 111–118 (2000)
Lawrence, H. J. et al. Mice bearing a targeted interruption of the homeobox gene HOXA9 have defects in myeloid, erythroid, and lymphoid hematopoiesis. Blood 89, 1922–1930 (1997)
Chase, A. et al. Fusion of ETV6 to the caudal-related homeobox gene CDX2 in acute myeloid leukemia with the t(12;13)(p13;q12). Blood 93, 1025–1031 (1999)
Kyba, M., Perlingeiro, R. C. & Daley, G. Q. HoxB4 confers definitive lymphoid-myeloid engraftment potential on embryonic stem cell and yolk sac hematopoietic progenitors. Cell 109, 29–37 (2002)
Joly, J. S. et al. Expression of a zebrafish caudal homeobox gene correlates with the establishment of posterior cell lineages at gastrulation. Differentiation 50, 75–87 (1992)
Kingsley, P. D. et al. Subtractive hybridization reveals tissue-specific expression of ahnak during embryonic development. Dev. Growth Differ. 43, 133–143 (2001)
Acknowledgements
We would like to thank members of the Zon laboratory, B. Paw and S. Orkin for critical reading of this manuscript. We also thank K. Humphries for HoxB4 retrovirus, H. G. Frohnhöfer for kgg mutants, J. Postlethwait and A. Amores for genomic sequences, and members of the zebrafish community for gifts of cDNAs. L.I.Z is an Investigator of the Howard Hughes Medical Institute. This work was supported by Legal Sea Foods, the Grousbeck family and grants from the NIH.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare that they have no competing financial interests.
Supplementary information
Rights and permissions
About this article
Cite this article
Davidson, A., Ernst, P., Wang, Y. et al. cdx4 mutants fail to specify blood progenitors and can be rescued by multiple hox genes. Nature 425, 300–306 (2003). https://doi.org/10.1038/nature01973
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1038/nature01973
This article is cited by
-
Adar-mediated A-to-I editing is required for embryonic patterning and innate immune response regulation in zebrafish
Nature Communications (2022)
-
A Rationale for the Use of Clotted Vertebral Bone Marrow to Aid Tissue Regeneration Following Spinal Surgery
Scientific Reports (2020)
-
Evolution of the bilaterian mouth and anus
Nature Ecology & Evolution (2018)
-
Role of HOXA9 in leukemia: dysregulation, cofactors and essential targets
Oncogene (2016)
-
Cdx ParaHox genes acquired distinct developmental roles after gene duplication in vertebrate evolution
BMC Biology (2015)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.