Abstract
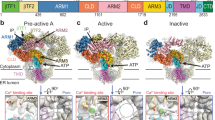
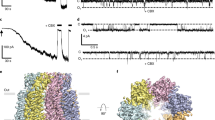
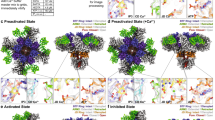
In a variety of cells, the Ca2+ signalling process is mediated by the endoplasmic-reticulum-membrane-associated Ca2+ release channel, inositol 1,4,5-trisphosphate (InsP3) receptor (InsP3R)1. Being ubiquitous and present in organisms ranging from humans to Caenorhabditis elegans, InsP3R has a vital role in the control of cellular and physiological processes as diverse as cell division, cell proliferation, apoptosis, fertilization, development, behaviour, memory and learning2. Mouse type I InsP3R (InsP3R1), found in high abundance in cerebellar Purkinje cells, is a polypeptide with three major functionally distinct regions: the amino-terminal InsP3-binding region, the central modulatory region and the carboxy-terminal channel region2. Here we present a 2.2-Å crystal structure of the InsP3-binding core of mouse InsP3R1 in complex with InsP3. The asymmetric, boomerang-like structure consists of an N-terminal β-trefoil domain and a C-terminal α-helical domain containing an ‘armadillo repeat’-like fold. The cleft formed by the two domains exposes a cluster of arginine and lysine residues that coordinate the three phosphoryl groups of InsP3. Putative Ca2+-binding sites are identified in two separate locations within the InsP3-binding core.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Berridge, M. J., Lipp, P. & Bootman, M. D. The versatility and universality of calcium signalling. Nature Rev. Mol. Cell Biol. 1, 11–21 (2000)
Furuichi, T. & Mikoshiba, K. Inositol 1,4,5-trisphosphate receptor-mediated Ca2+ signaling in the brain. J. Neurochem. 64, 953–960 (1995)
Uchiyama, T., Yoshikawa, F., Hishida, A., Furuichi, T. & Mikoshiba, K. A novel recombinant hyperaffinity inositol 1,4,5-trisphosphate (IP3) absorbent traps IP3, resulting in specific inhibition of IP3-mediated calcium signaling. J. Biol. Chem. 277, 8106–8113 (2002)
Overduin, M., Cheever, M. L. & Kutateladze, T. G. Signaling with phoshoinositides: better than binary. Mol. Interventions 1, 151–159 (2001)
Hamada, K., Shimizu, T., Matsui, T., Tsukita, S. & Hakoshima, T. Structural basis of the membrane-targeting and unmasking mechanisms of the radixin FERM domain. EMBO J. 19, 4449–4462 (2000)
Ferguson, K. M., Lemmon, M. A., Schlessinger, J. & Sigler, P. B. Structure of the high affinity complex of inositol trisphosphate with a phospholipase C pleckstrin homology domain. Cell 83, 1037–1046 (1995)
Hyvonen, M. et al. Structure of the binding site for inositol phosphates in a PH domain. EMBO J. 14, 4676–4685 (1995)
Hotoda, H. et al. Molecular recognition of adenophostin, a very potent Ca2+ inducer, at the d-myo-inositol 1,4,5-trisphosphate receptor. Biochemistry 38, 9234–9241 (1999)
Murzin, A. G., Lesk, A. M. & Chothia, C. beta-Trefoil fold. Patterns of structure and sequence in the Kunitz inhibitors interleukins-1β and 1α and fibroblast growth factors. J. Mol. Biol. 223, 531–543 (1992)
Holm, L. & Sander, C. Protein structure comparison by alignment of distance matrices. J. Mol. Biol. 233, 123–138 (1993)
Huber, A. H., Nelson, W. J. & Weis, W. I. Three-dimensional structure of the armadillo repeat region of β-catenin. Cell 90, 871–882 (1997)
Cingolani, G., Petosa, C., Weis, K. & Muller, C. W. Structure of importin-β bound to the IBB domain of importin-α. Nature 399, 221–229 (1999)
Peifer, M., Berg, S. & Reynolds, A. B. A repeating amino acid motif shared by proteins with diverse cellular roles. Cell 76, 789–791 (1994)
Rost, B., Sander, C. & Schneider, R. PHD—an automatic mail server for protein secondary structure prediction. Comput. Appl. Biosci. 10, 53–60 (1994)
Jones, D. T. GenTHREADER: an efficient and reliable protein fold recognition method for genomic sequences. J. Mol. Biol. 287, 797–815 (1999)
Hamada, K., Miyata, T., Mayanagi, K., Hirota, J. & Mikoshiba, K. Two-state conformational changes in inositol 1,4,5-trisphosphate receptor regulated by calcium. J. Biol. Chem. 277, 21115–21118 (2002)
Jiang, Q. X., Thrower, E. C., Chester, D. W., Ehrlich, B. E. & Sigworth, F. J. Three-dimensional structure of the type 1 inositol 1,4,5-trisphosphate receptor at 24 Å resolution. EMBO J. 21, 3575–3581 (2002)
Wilcox, R. A., Primrose, W. U., Nahorski, S. R. & Challiss, R. A. New developments in the molecular pharmacology of the myo-inositol 1,4,5-trisphosphate receptor. Trends Pharmacol. Sci. 19, 467–475 (1998)
Kozikowski, A. P., Ognyanov, V. I., Fauq, A. H., Nahorski, A. R. & Wilcox, R. A. Synthesis of 1d-3-deoxy-, 1d-2,3-dideoxy-, and 1d-2,3,6-trideoxy-myo-inositol 1,4,5-trisphosphate from quebrachitol, their binding affinities, and calcium release activity. J. Am. Chem. Soc. 115, 4429–4434 (1993)
Yoshikawa, F. et al. Mutational analysis of the ligand binding site of the inositol 1,4,5-trisphosphate receptor. J. Biol. Chem. 271, 18277–18284 (1996)
Marchant, J. S. & Taylor, C. W. Cooperative activation of IP3 receptors by sequential binding of IP3 and Ca2+ safeguards against spontaneous activity. Curr. Biol. 7, 510–518 (1997)
Sienaert, I. et al. Molecular and functional evidence for multiple Ca2+-binding domains in the type 1 inositol 1,4,5-trisphosphate receptor. J. Biol. Chem. 272, 25899–25906 (1997)
Sienaert, I. et al. Localization and function of a calmodulin/apocalmodulin binding domain in the N-terminal part of the type 1 inositol 1,4,5-trisphosphate receptor. Biochem. J. 365, 269–277 (2002)
Yoshikawa, F. et al. High efficient expression of the functional ligand binding site of the inositol 1,4,5-triphosphate receptor in Escherichia coli. Biochem. Biophys. Res. Commun. 257, 792–797 (1999)
Van Duyne, G. D., Standaert, R. F., Karplus, P. A., Schreiber, S. L. & Clardy, J. Atomic structures of the human immunophilin FKBP-12 complexes with FK506 and rapamycin. J. Mol. Biol. 229, 105–124 (1993)
Pflugrath, J. W. The finer things in X-ray diffraction data collection. Acta Crystallogr. D 55, 1718–1725 (1999)
Terwillinger, T. C., Kim, S. H. & Eisenberg, J. Generalized method of determining heavy-atom positions using the difference Patterson function. Acta Crystallogr. A 43, 1–5 (1987)
La Fortelle, E. D. & Bricogne, G. Maximum-likelihood heavy atom parameter refinement in the MIR and MAD methods. Methods Enzymol. 276, 472–494 (1997)
Brunger, A. T. et al. Crystallography and NMR system: A new software suite for macromolecular structure determination. Acta Crystallogr. D 54, 905–921 (1998)
Jones, T. A., Zou, J. Y., Cowan, S. W. & Kjeldgaard, M. Improved methods for binding protein models in electron density maps and the location of errors in these models. Acta Crystallogr. A 47, 110–119 (1991)
Acknowledgements
We thank A. Nakagawa, A. Miyazaki and K.T. Chong for their assistance at the beamline BL44XU at SPring-8, Japan; the staff at the X8-C and X25 beamline of Brookhaven National Laboratory and the BioCars beamline at Advanced Photon Source for their assistance; D. Jones and M. Swindells for mGenTHREADER analysis; and members of our division for discussions. This work was supported by grants from the Canadian Institutes of Health Research (CIHR) to I.B. and M.I., and by grants from RIKEN (to K.M.) and the Ministry of Education, Science, Sports, and Culture of Japan (to K.M. and T.M.). M.I. is a CIHR Investigator.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare that they have no competing financial interests.
Supplementary information
Rights and permissions
About this article
Cite this article
Bosanac, I., Alattia, JR., Mal, T. et al. Structure of the inositol 1,4,5-trisphosphate receptor binding core in complex with its ligand. Nature 420, 696–700 (2002). https://doi.org/10.1038/nature01268
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nature01268
This article is cited by
-
Substrate promiscuity of inositol 1,4,5-trisphosphate kinase driven by structurally-modified ligands and active site plasticity
Nature Communications (2024)
-
Structural titration reveals Ca2+-dependent conformational landscape of the IP3 receptor
Nature Communications (2023)
-
Candesartan, an angiotensin-II receptor blocker, ameliorates insulin resistance and hepatosteatosis by reducing intracellular calcium overload and lipid accumulation
Experimental & Molecular Medicine (2023)
-
Biological functions and potential therapeutic applications of huntingtin-associated protein 1: progress and prospects
Clinical and Translational Oncology (2022)
-
Exploration of inositol 1,4,5-trisphosphate (IP3) regulated dynamics of N-terminal domain of IP3 receptor reveals early phase molecular events during receptor activation
Scientific Reports (2019)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.