Abstract
The prefrontal cortex (PFC), seat of the highest-order cognitive functions, constitutes a conglomerate of highly specialized brain areas and has been implicated to have a role in the onset and installation of various neurodevelopmental disorders. The development of a properly functioning PFC is directed by transcription factors, guidance cues and other regulatory molecules and requires the intricate and temporal orchestration of a number of developmental processes. Disturbance or failure of any of these processes causing neurodevelopmental abnormalities within the PFC may contribute to several of the cognitive deficits seen in patients with neurodevelopmental disorders. In this review, we elaborate on the specific processes underlying prefrontal development, such as induction and patterning of the prefrontal area, proliferation, migration and axonal guidance of medial prefrontal progenitors, and their eventual efferent and afferent connections. We furthermore integrate for the first time the available knowledge from genome-wide studies that have revealed genes linked to neurodevelopmental disorders with experimental molecular evidence in rodents. The integrated data suggest that the pathogenic variants in the neurodevelopmental disorder-associated genes induce prefrontal cytoarchitectonical impairments. This enhances our understanding of the molecular mechanisms of prefrontal (mis)development underlying the four major neurodevelopmental disorders in humans, that is, intellectual disability, autism spectrum disorders, attention deficit hyperactivity disorder and schizophrenia, and may thus provide clues for the development of novel therapies.
Similar content being viewed by others
The prefrontal cortex in neurodevelopmental disorders
Neurodevelopmental disorders affect a large percentage of the population worldwide. Although the available drugs can alleviate some of the symptoms associated with these disorders, they are not curative and adverse drug reactions are often observed. In addition, many neurodevelopmental disorder-associated symptoms, especially cognitive symptoms, still cannot be treated effectively. To improve the prognosis of a given neurodevelopmental disorder, the effectiveness of existing therapies and the potential for finding new treatment strategies, detailed knowledge of the development and pathophysiology of the disorders is mandatory.1, 2 Neurodevelopmental disorders such as intellectual disability (ID), autism spectrum disorders (ASDs), attention deficit (hyperactivity) disorder (AD(H)D) and schizophrenia share particular cytoarchitectonical, connectional and functional features suggesting a similar neurodevelopmental origin. Unfortunately, for the most part, detailed molecular studies of developmental events within brain areas that are involved in the etiology of these neurodevelopmental disorders are still lacking.
A wealth of data indicates that the prefrontal cortex (PFC) contributes to the cognitive deficits or endophenotypes of many, if not all, neurodevelopmental disorders.3, 4, 5, 6, 7, 8, 9, 10, 11, 12 As a conglomerate of individually unique subareas, the PFC has a key role in the execution of higher-order cognitive functions, for example, language comprehension and cognitive functions involved in decision making such as planning and reasoning.13, 14, 15, 16 In this respect, the different subareas within the PFC mediate various processes including response inhibition, working memory, attention or autonomic control.17, 18, 19, 20 Furthermore, the medial regions of the PFC, the mPFC, such as the infralimbic, prelimbic and cingulated areas, have a role in the cognitive deficits of many neurodevelopmental disorders.7, 11
The main neurodevelopmental disorders—ID, ASDs, AD(H)D and schizophrenia—have a complex etiology involving a large number of genes and environmental factors that also affect prefrontal brain regions, including those of the mPFC. Although multiple genes have been found to be associated with each of these disorders, the actual function and involvement of individual genes in the developmental aspects of mPFC formation in particular are largely unknown. Abnormalities in the expression of these genes often lead to impaired or deviant functioning of several brain structures, including the mPFC, affecting behavior as previously shown in animal studies.21, 22
In the following, we will give an overview of the main neurodevelopmental disorders with a particular focus on the defects in the development of the mPFC, bearing in mind that areas other than the mPFC may also contribute to the etiology of the disorders.
ID
The diagnostic category mental retardation groups a number of syndromes with severe ID that are associated with chromosomal abnormalities such as Down Syndrome (trisomy of chromosome 21), Prader–Willi and Angelman Syndromes, Williams–Beuren Syndrome, Smith–Magenis Syndrome, DiGeorge Syndrome and monosomy of chromosome 1p36.1.23, 24, 25, 26 Other ID syndromes show mild-to-moderate phenotypes and are associated with mutations, small insertions/deletions or copy number variations affecting a single gene, for example, fragile X syndrome, caused by a mutation in the FMR1 gene27, 28 and Kleefstra syndrome, caused by a functional loss of the EHMT1 gene.29 Most ID syndromes are associated with developmental deficits in general, including distorted development of the mPFC.23, 24, 26, 30 In this respect, during the development of the mPFC of ID patients, molecular/cellular defects have been shown to occur in (a) the proliferation of neuronal progenitor cells,31, 32 (b) migration of cortical neurons33, 34, 35, 36, 37 and (c) synaptogenesis.32, 38, 39
ASDs
The ASDs include autism, Asperger’s syndrome and ‘pervasive developmental disorder not otherwise specified’ Diagnostic and Statistical Manual of Mental Disorders-5th edition (DSM-V). They constitute a group of wide-ranging neurodevelopmental disorders that are characterized by variable impairments in three core symptom domains, that is, reciprocal social interaction, (verbal and nonverbal) communication, and restricted, repetitive and stereotyped patterns of behavior, interests and activities.40, 41, 42, 43 Although many of these behavioral impairments are driven by deficits in basal ganglia and amygdala functioning, cognitive dysfunctions such as memory deficits and deficits in social interaction and perception are integrated by the mPFC.44 The neurodevelopmental basis underlying the defects in language and speech, which are often part of the diagnosis in ASDs relates to abnormalities in fronto-striatal functioning.45, 46, 47, 48, 49 Regarding the development of the mPFC of ASD patients, molecular/cellular defects have been reported to occur in (a) the proliferation of neuronal progenitor cells50, 51 resulting in macrocephalic and minicolumn pathology in several brain areas including the PFC,3, 40, 42, 52, 53, 54 (b) migration and differentiation of GABA ergic parvalbumin+ (PV+) interneurons toward the PFC,36, 55, 56 (c) axon guidance, as there seems to be a disconnection of long-distance axonal pathways57, 58 and (d) synaptogenesis, particularly of GABAergic synapses.59, 60, 61 Deficits in integration and early information processing can be explained by hyperconnectivity combined with slower synapses.62 Furthermore, there is evidence for amplified activation and density of microglia within the PFC of ASD patients.57, 63, 64
AD(H)D
Inattention, hyperactivity/impulsivity and motivational/emotional dysregulation are the core symptom domains in AD(H)D. In AD(H)D patients, the mPFC-directed cognitive functions are affected and frequently of early onset.65, 66, 67 A delay in cortical maturation specifically in the most prefrontal areas and its connections to other brain areas has often been observed68 and there is increasing evidence that glutamate signaling is affected.69 During development, the PFC of patients with AD(H)D shows molecular/cellular defects in (a) the white matter, suggesting axon guidance deficits70, 71, 72 (b) dopaminergic and noradrenergic connectivity with the cerebellum and striatum65, 67, 73, 74, 75, 76 and (c) synaptogenesis influencing the electrophysiological properties and functioning of PFC neurons.77, 78, 79
Schizophrenia
Schizophrenia is thought to affect mainly (social) cognition, but it usually is also associated with chronic problems of behavioral and emotional regulation.80 Schizophrenia is characterized by a breakdown of thought processes manifested as delusions and hallucinations (positive symptoms) and by poor emotional responsiveness, and disorganized thinking and speech (negative symptoms). People with schizophrenia are likely to have co-morbidities such as major depression and anxiety disorders. Furthermore, working and long-term memory, attention, executive functioning and speed of processing are often affected.80 All of these symptoms can at least to some extent be linked to (impaired) PFC functioning.5, 12, 81, 82, 83, 84 During development of the mPFC in schizophrenia patients, molecular/cellular defects may occur in the (a) proliferation of neuronal progenitor cells, as reflected by the observed severely decreased gray-matter volume,85 as well as of GABAergic PV+ interneurons,86, 87 (b) postnatal pruning of dendritic trees and synapse loss,88, 89, 90, 91 (c) general connectivity of various neurotransmitter systems such as the glutamate, GABA and dopamine systems together with a reduced connectivity with other cortical areas.92, 93, 94, 95, 96, 97, 98, 99
Rodent models of neurodevelopmental disorders
Before one can start to develop better and more target-specific therapies for patients with neurodevelopmental disorders, it is necessary to first unravel elementary processes of brain development in adequate animal models and to understand subsequent developmental processes in those areas associated with the endophenotypes of neurodevelopmental disorders. In this way, fundamental hypotheses can be created and tested in relation to the etiology of these disorders. Such parallel approaches are crucial to eventually design optimal treatment strategies.
As mentioned before, although the PFC is often referred to as a single brain region, many subdivisions into distinct areas can be made, each of which possesses its own specific cytoarchitecture, cytochemistry, connectivity and functional properties. Defining these areas across species suffers from the fact that large interspecies differences exist in the layering per area, fueling the debate on whether or not rodents possess a region equivalent to the human PFC as they lack a granular zone in this area.100, 101 However, it should be noted that the formation of the general laminar pattern in the PFC shows a relation with phylogenesis: in ‘higher’ mammalian species, such as primates and humans, PFC regions can be granular, that is, they possess a granular layer IV, as well as an agranular layer. The ‘lower’ the species, the smaller the proportion of granular PFC regions (for reviews, see refs 100, 101). Thus the concept of homologous structures with similar functions may apply.
In this review, we will focus on the rodent mPFC and its structure–function relationships with connected brain areas in the context of neurodevelopmental disorders.102, 103 One example of a well-defined rodent model for neurodevelopmental disorders is the apomorphine-susceptible and apomorphine-unsusceptible Wistar rat. The behavioral impairments seen in the apomorphine-susceptible rats resemble features of schizophrenia.104, 105, 106 At least part of this phenotype can be attributed to the differences in the mesocorticolimbic projections.107
Furthermore, mouse models are ideally suited to study targeted molecular alterations.102, 108, 109, 110, 111, 112, 113, 114 In this way, genetic variants identified through association studies can be tested for their biological function and correlated with cognitive endophenotypes of human neurodevelopmental disorders. However, the traditional techniques of targeted mutation used in these kinds of model systems are systemic in nature and often result in inducing compensation mechanisms. Cre-Lox and knock-in systems still affect a large part of the brain, but can offer cell-type selective and temporally controlled strategies to achieve targeted mutations at different pre- and postnatal ages.115 Although in utero electroporation-mediated gene transfer spatially restrict gene repression or genetic rescues to early developmental time-points (app. E10-E17), virally mediated gene transfer can be performed pre- as well as postnatally.116 Furthermore, intersectional genetics (Flpe/Cre) to selectively mutate genes of interest in overlapping areas between a Cre and a Flpe allele (for example, Dlx5 Flpe and a region-specific Cre to selectively target GABAergic interneurons in a region of interest) increases the spatial selectivity of such approaches. Using these techniques, it is possible to knock down or rescue a particular gene in a specific part of the brain (for example, PFC) and at a specific time during brain development.
By employing various behavioral tasks, it is now possible to specifically test endophenotypes associated with mPFC function in rodent models, such as working memory, conditioned associative learning, attentional set shifting and reversal learning.117, 118, 119, 120, 121, 122 Consequently, by combining the targeted mutation with specific behavioral tests and instead of having to study a particular disease as a whole, one can now molecularly unravel the individual cognitive endophenotypes.21, 22 A further advantage of such an approach is that a causal inference can be made between the expression of a particular gene in a specific brain locus and one or more cognitive (endo)phenotypes, which is not yet possible in humans.
Developmental aspects of PFC formation
The PFC represents the functionally most advanced brain area with the longest period of maturation. This maturation includes proliferation and migration of neurons, growth of dendrites, the formation of neural micro- and macro-circuits through efferent/afferent axonal projections, and the fine-tuning of synaptic contacts and neuronal density steered by experience. This maturation process starts with an initial phase of cell division within an intrinsically specified PFC region, in which specific transcription factors (TFs) have a timing-critical role (Figure 1). Developmental events such as induction, migration and axon guidance are under the control of extrinsic cues and sculpt the identity of frontal areas. Appropriate cognitive behavior is fine-tuned over time by activity-dependent processes including sensory stimuli and social interactions, which in turn leads to pruning and cell death of unused connections.123 As a result, intricate convergence of connections with various other brain areas occurs, eventually creating the unique identity of the PFC and the subareas it encompasses (Figure 1). Here, the initial focus will be on the early developmental events of the (fore)brain as a whole and the molecules that are relevant during this phase. Although little is known about the early developmental characteristics of the PFC, many early principles and main mechanisms of forebrain compartmentalization and maturation are also applicable to PFC development. Important to keep in mind is the influence of external stimuli (for example, stress, drugs and hormones) that, if excessive, can lead to an altered development of the PFC and its connected areas.123 Thus, the knowledge about the genes that are involved in the structural and functional development of the (fore)brain and in particular the PFC is important for a better understanding of the molecular mechanisms underlying (disturbed) cognitive functions. Eventually, this knowledge may enable us to therapeutically intervene when this ‘developmental balance’ is shifted toward neuropsychiatric disorder.
Bird’s eye view of developmental events required for prefrontal cortex (PFC) formation. The identity of the PFC is sculpted over time by intrinsic developmental mechanisms such as expansion by proliferation and regional specification by the differential expression of intrinsic factors (e.g., transcription factors), indicated in blue. These intrinsic factors can control genes (transcriptional control) that affect other developmental events such as the expression and release of soluble morphogens, migration of neurons or guidance molecules that direct axons from other brain areas towards the PFC and vice versa to establish appropriate connectivity. These extrinsic factors are depicted in red. Pruning of appropriate connections and neuron death are under the control of external stimuli (green).
Induction of (pre)frontal boundaries
The developmental progression of the forebrain starts with regional expansion through division of neuronal progenitor cells in proliferative zones lining the embryonic ventricles of the brain. The most anterior part of the neural tube develops into three primary vesicles even before the posterior section of the tube has formed: the prosencephalon (forebrain), mesencephalon (midbrain) and rhombencephalon (hindbrain).124 After closure, the neural tube is characterized by a sequence of swellings and constrictions along the anteroposterior axis, some of which subsequently develop into strict boundaries.125
Except for the specific boundary compartment, the zona limitans intrathalamica (ZLI), no unique set of boundary markers has been identified for regions of the forebrain and most of the telencephalon develops in an unsegmented way.125 Anterior of the midbrain–hindbrain border (MHB) or isthmus, the diencephalon consists of three neuromeres (p1–p3) according to the so-called prosomeric model.125, 126, 127 The more anterior prosomeres (p4–p6) subdivide the secondary prosencephalon (hypothalamus and telencephalon).128 The boundaries that are created function to arrange and stabilize local signaling centers or ‘organizers’ important for the early patterning of the embryonic brain (Figures 2a and b). Gradually, gradients of soluble morphogens and growth factors (Fgfs, BMPs, SHH and Wnts)129, 130 are secreted from signaling centers and regulate the graded expression of certain intrinsic TFs, a process that is called induction131 (Figures 2a and b).
Molecular stages in the development of the PFC. (a) Schematic representation of the frontal view of a young (E11.5) mouse forebrain showing inductive influences (morphogens such as Fgfs, Wnts, SHH and BMPs; stage I). (b) Sagittal schematic views. These morphogens (stage I) have an effect on regional specification through intrinsic expression of transcription factors (stage II). This combinatorial code will have its effect on the cell-type specification of the major neurotransmitter systems (stage III). The neurotransmitter systems will connect to the PFC, shaping it and establishing the respective neural networks (stage IV). ANR, anterior neural ridge; DA, dopaminergic; DI, diencephalon; MES, mesencephalon; MET, metencephalon; MHB, mid-hindbrain border; NA, noradrenergic; PFC, prefrontal cortex; RPC, rostral patterning center; SHH, sonic hedgehog; Tel, telencephalon; VSC, ventral signaling center; ZL, zona limitans; 5-HT, serotonergic.
Fgfs, especially Fgf8, Fgf17 and Fgf18 from the rostral patterning center (also called anterior neural ridge) provide, apart from their role in other areas, positional information on the presumptive prefrontal region along the rostro-caudal axis of the forebrain.132, 133 The dorsal patterning center or cortical hem secretes Bmp4/Wnt3A, which has a role in medial and dorsal pallium patterning,134, 135, 136 but in combination with SHH also steers prefrontal formation (Figures 2a and b). SHH is expressed by the ventral signaling center and regulates Fgf8 expression through the transcriptional repressor Gli3.137, 138, 139, 140 Absence of Fgf17 leads to a reduced PFC size and abnormal social behavior.141, 142 Thus, Bmp, Wnt and Fgf proteins all work coordinately to pattern the most rostral telencephalon.139, 143 Interference with each of the three Fgf receptor subtypes results in reduced numbers of either excitatory or inhibitory neurons, specifically in the prefrontal area and often resulting in altered behavior.144, 145, 146, 147, 148, 149
Regional identity of the PFC through intrinsic patterning
The gradients of morphogens and signaling molecules from the early patterning centers impart positional information influencing the expression of intrinsic TFs (Figure 2b). These have a crucial role in the regionalization of the forebrain and correlate with morphologic boundaries, the so-called regional specification underlying the spatio-temporal control of postnatal arealization.131, 150, 151, 152 The regional identity that is created by the expression of TFs includes the final cell-type specification.153 The inductive signals provided by morphogens and signaling molecules regulate the combinatorial expression of TFs and other regulatory factors, resulting in the generation of specific neuronal subtypes154, 155 (Figure 2a and b).
The interaction between extrinsic growth factors and intrinsic TFs during the early developmental events evolves through rostral patterning by the factors Fgf8 and Fgf17 through the Fgf receptors. This Fgf-signaling promotes the expression of the TFs Foxg1, Six3, Sp8, Pax6, Erm (etv5), Er81 (etv1), Nkx2.1 and Pea3, and represses the expression of Coup-tf1 and Emx2 more caudally.131, 133, 156 Although it is most likely the expression of a combination of multiple TFs that underlies the identity of an area, there are a few individual TFs that are specifically linked to the development of the most rostral part of the cortex. The expression of the TFs Pax6 and Emx2, for example, is known to have a role in cortical identity in general.131, 157, 158 Yet, very few TFs are specifically expressed in and linked to early PFC development.
During the course of development, distinct neuronal cell types will express a variety of proteins that are involved in migration, targeting (for example, axon guidance) and specific neurotransmitter release. This set of proteins is unique for each cell type, thereby regulating the formation of functional areas.159 The expression of the respective genes (extrinsic genes) is under the control of a distinct combinatorial code of TFs generating neuronal diversity160(Figure 1 and Figure 2). Other TFs such as Rest4 and Nurr1 display increased expression in the PFC and are involved in various aspects of cognitive behavior.161, 162 Although an abundance of genome-wide expression data shows that specific TFs are expressed in later stages of PFC development, their downstream targets and functional relevance are largely unknown.163, 164, 165, 166 In fact, the existing data are now congruent with a model in which each neuronal cell type within the PFC (but also other areas) most likely uses an exclusive code of intrinsic genes to control the expression of extrinsic genes. This code is unique to each particular cell type essential for the sequential steps in development. The next level of complexity starts off when extrinsic mechanisms such as migration and afferent input begin to have a role in the development of the prefrontal areas.
Proliferation and migration of PFC neurons
The PFC, like other cortical areas, expands by generating new neurons through (a)symmetric divisions of radial glia cells in the (sub)ventricular zone lining the ventricles.167, 168 During this process, reduction of the extrinsic morphogen Fgf8 results in less proliferation and more apoptosis, which ultimately changes the identity of the cortex.132, 169, 170 In particular Fgf has a determining role in the production of excitatory glutamatergic pyramidal neurons in the most anterior part of the cortex with deletion of the gene resulting in a reduced number of excitatory cortical neurons.171 Many TFs controlling the cell cycle, including cyclinD1, drive prefrontal expansion.39 Some newborn progenitors or intermediate progenitor cells expressing Tbr2 migrate to the subventricular zone to generate neurons. Lack of Tbr2 expression results in reduced cortical surface and thickness.172, 173, 174, 175 It is furthermore widely accepted that classical neurotransmitters such as dopamine and serotonin have an early role in controlling the neuron numbers within the PFC.176, 177, 178
The differential expression of TFs but also of adhesion and axon guidance molecules reflects a signage map for migrating neurons. The expression patterns are graded along the anterior–posterior and medial–lateral axes of the embryonic brain instructing neurons to establish functionally distinct lamina. During embryogenesis, most brain areas deploy radial migration in multiple waves as their major route to establish lamination within the structure.167, 179, 180 Radial glia cells, with their cell body within the ventricular zone, send out their glial processes toward the pial surface where they attach to the basal membrane. Newborn neurons that become (excitatory) projection neurons use the glial scaffold to migrate to their final place in the brain by using either somal translocation or locomotion.167, 180, 181 The ventricular zone generates the deeper layer neurons, including the subplate, layer VI and subsequently layer V projection neurons. Additionally, Cajal–Retzius neurons are generated within the cortical hem and to a lesser extent at other sites in the subpallium and septum. These layer I neurons express Reelin, a large secreted glycoprotein intricately involved in the inside-out laminar patterning of cortical neurons.182, 183 At later stages, the subventricular zone gives birth to neurons which migrate radially into the cortical plate past the deep layer neurons and form layers IV, III and II of the PFC, creating an inside-out pattern. Most of the projection neurons (80%) use glutamate as their neurotransmitter projecting to distant cortical and subcortical targets. The basic molecular developmental mechanisms that have been elucidated in rodent studies are in principle similar to those in humans, even though the human brain has gone through a series of additional evolutionary steps, including size, shape and gyrification modifications.184, 185, 186
Migration of GABAergic interneurons towards the PFC
A small proportion of neurons, which includes the majority of GABAergic (GAD65/67+) interneurons originating from the ganglionic eminences, migrate tangentially to the cortical plate, then radially to reach their target lamina.187 The subpallial interneurons migrate via a lengthy route towards the PFC using directional cues to eventually position themselves between pyramidal projection neurons on which they synapse.167, 188 Medial ganglionic eminence-derived interneurons will generate PV and somatostatin interneurons that populate all cortical structures (as well as hippocampus, striatum, amygdala, etc). These interneurons are specified in the medial ganglionic eminence by the expression of Nkx2.1 and Lhx6 followed by Sox6 expression as they start migrating. In contrast, caudal ganglionic eminence-derived interneurons encompass all 5-HT3A-expressing interneurons of various morphology and physiology.188 The homeobox TFs Dlx1 and Dlx2 mainly regulate the maturation of GABAergic (inter)neurons within the ganglionic eminences, having the TF Arx as a downstream target.133 However, the combinatorial expression of TFs such as Olig2, Dlx5, Arx, Lhx6, Cux2, NPAS1 and MafB define the various subpopulations of interneurons within the subpallium that end up in the (prefrontal) cortex.188, 189 As development progresses, interneurons within the (prefrontal) cortex start to express transporters (GAT-1 and -3), VGAT and components of GABAergic synapses190 making them highly adaptive to the maturing PFC.
Axon guidance, target selection and synapse formation of PFC neurons
The assembly of neuronal circuits during embryonic development relies upon the guidance of growing axons to their synaptic targets. To help them find their synaptic partners, developing axons are tipped with a highly motile sensory structure, the growth cone. Growth cones are instructed to follow predetermined trajectories by heterogeneously distributed guidance molecules in the extracellular environment. Binding of axon guidance molecules to receptor complexes on the growth cone surface initiates intracellular signaling events, which modulate growth cone morphology and directionality through local modifications of the cytoskeleton. Axon guidance molecules can act as attractants or repellents, that is, either directing growth cones toward a specific structure or preventing them from entering inappropriate regions. Furthermore, these cues exist as membrane-associated molecules acting at short ranges or as soluble agents with long-distance effects.191, 192, 193, 194 The responses of growing axons to particular cues, however, may change as they grow toward their final targets.176 For example, Semaphorin 3F is such a bidirectional guidance cue that, through binding with Neuropilin-2, initially repels dopaminergic axons from the rostral ventral tegmental area on their way to the mPFC, and later attracts and orients them within the mPFC.176 When the axonal growth cone has been guided to the proper target, synaptic contacts can be formed that are mediated by adhesion molecules such as the cadherins.195, 196 Newly formed synaptic contacts change their functional properties as development progresses and contribute to the maturation and functioning of an area.197, 198 Furthermore, the immature afferent projections are refined via the same guidance molecules in topography (pruning of branches), convergence (less efferent projections onto one cell) as well as postsynaptic compartment (less afferent dendritic innervation) in specific brain areas.197, 198, 199 Changes occurring in pyramidal morphology in terms of expansion of dendritic complexity are specifically apparent in layer III.200 Furthermore, during the first four postnatal weeks the local inhibitory interneuron networks in the mPFC undergo an extensive process of maturation, both at the level of intrinsic functional as well as network properties.201, 202 Given that inhibitory network activity is thought to contribute to the proper construction of cortical networks, the refinement of synaptic connectivity in inhibitory and excitatory networks leads to developmental plasticity and fine-tuning of complex behavior.
Topographic map formation in PFC connectivity: parcellation versus lamination
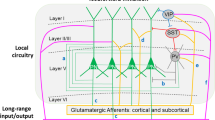
As mentioned above, in rodents and other phylogenetically ‘higher’ species, the PFC is not one homogeneous cortical region but is compartmentalized into a number of structurally and functionally distinct prefrontal areas, each of which is thought to possess characteristic input–output profiles. In general, the rodent PFC can be subdivided into medial, lateral and ventral sections. Within the medial portion, the anterior cingulate (Cg), prelimbic (PL) and infralimbic (IL) cortices (Figure 3) and dorsal peduncular cortex can be distinguished from dorsal to ventral.203 The lateral and ventral PFC consists of the orbitofrontal cortex and the agranular insular cortices.204 The different areas of the PFC are connected to various other brain regions through highly organized projections controlling decision-directed behavior.205, 206, 207
Neurodevelopmental disorder-associated genes that are involved in mPFC development. Various genes are associated with neurodevelopmental events in the mPFC (proliferation, migration, guidance targeting and connectivity) of which some can also be found in association studies with the four major neurodevelopmental disorders ID, ASDs, AD(H)D, schizophrenia. The letter size in the ‘cloud’ of genes is indicative of the frequency of the gene associated with the various neurodevelopmental disorders connected to that particular neurodevelopmental event. Cg, cingulate cortex; CP, cortical plate; DN, dividing neuroblast; GC, growth cone; IL, infralimbic cortex; IN, interneuron; IPC, intermediate progenitor; IZ, intermediate zone; MN, migrating neuron, PN, post-mitotic neuron; PrL, prelimbic cortex; PZ, proliverative zone; RG, radial glia; (1) Commissural and corticocortical projection neurons, respectively; (2) subcerebral projection neurons to basal ganglia, diencephalon, midbrain, hindbrain and spinal cord; (3) corticothalamic projection neurons to mediodorsal thalamic targets; (2) and (3)=corticofugal.
Input connectivity of the mPFC
In terms of the afferent connectivity of the mPFC, a comprehensive and detailed comparison of area-specific input connectivity is still lacking. The mPFC is known to receive long ascending projections from the ventral hippocampus,208, 209 from cholinergic neurons of the basal forebrain,210, 211 from dopaminergic neurons of the rostral part of the medial ventral tegmental area176, 212, 213 and from serotonergic/cholinergic neurons of the brainstem along a highly defined trajectory.214, 215 Functionally, the connection with the ventral hippocampus is thought to be of particular importance for the functioning of the mPFC during cognitive tasks.216, 217 The cholinergic and dopaminergic systems are considered to modulate mPFC activity and attentional performance.218, 219 Interestingly, the dopaminergic projections from the ventral tegmental area show strong laminar and cell-type specificity. They form dense contacts exclusively with interneurons in layers V and VI,176, 213, 220, 221 while for example projections from limbic and thalamic regions innervate both PV+ interneurons and pyramidal cells throughout layers II–VI.222, 223, 224 Furthermore, connections of the mPFC with both the basolateral amygdala209, 225 and the striatum are implicated in motivated behavior.226, 227 Interestingly, the long-range connections originating from the basolateral amygdala have been shown to not only be layer- but also cell-type specific. Neurons in the basolateral amygdala preferentially target layer II pyramidal neurons in the mPFC, such as PL, and amygdala, with which they can form reciprocal connections.225, 228
Output connectivity of the mPFC
As in other cortical areas, the long-range efferent connections of the PFC are mediated by excitatory projection neurons, that is, glutamatergic pyramidal cells. Depending on the PFC area, the pyramidal cells project to many structures such as the basal forebrain, olfactory and cortical structures, amygdala, striatum, (hypo)thalamus and the brainstem.204, 215, 225, 226, 229 In addition, prefrontal pyramidal neurons project to various subcortical areas thereby modulating dopaminergic, adrenergic, cholinergic and serotonergic projection systems.101, 204 The targets of the projection neurons show distinct layer specificity. Layer III pyramidal neurons connect the mPFC mainly to other cortical areas, whereas layers V and VI pyramidal cells project primarily to subcortical targets.230, 231 Furthermore, there is evidence for layer specificity of projections onto individual subcompartments of single brain structures. In terms of the nucleus accumbens, mPFC layer II pyramidal neurons preferentially innervate the core region, whereas neurons of deep layers V and VI innervate the core as well as the shell region.232
In contrast to the input connectivity, there is ample data demonstrating that the output connectivity properties of the mPFC are area dependent, which supports the notion that prefrontal areas are involved in modulating various aspects of cognitive behavior,203, 204, 229 not only in rodents but also in a number of other species.220, 229, 230 The dorsomedial areas of the PFC establish connections with the sensorimotor and association cortex, which are lacking in the ventral parts of the PFC. The ventral parts, however, establish relatively strong connections with the amygdaloid complex and limbic association cortices. Furthermore, the IL has been shown to mainly project to autonomic/visceral related sites, supporting its role in visceromotor activity,204 whereas the PL primarily innervates limbic sites that are thought to affect cognition.
Future translational avenues of research
In summary, substantial progress has been made in the past decades toward understanding the etiology of neurodevelopmental disorders at the molecular, cellular and systems levels. Nevertheless, we have only just begun to thoroughly study the development of a conglomerate of specific brain areas that as a group define the PFC and that are involved in the etiology of these disorders. In this context, it is remarkable that the exact molecular orchestration of the development of the PFC is still largely unknown. What are the molecular mechanisms that create a correctly parcellated and layered PFC? How are the extensive and highly specific interactions between various signaling pathways that are connecting the individual areas fine-tuned and how can we manipulate these? We are also only beginning to shed light on the large variety of neuronal cells and their integration in prefrontal local and global networks, let alone that we would know all the molecules that guide their differentiation and projections.
To test targeted molecular variations, rodents have emerged as an excellent model. Animal models and functional assays are invaluable as it comes to decipher the exact functions of the large number of genes that are involved in the various aspects of PFC development, that is, induction of prefrontal boundaries, intrinsic patterning of the PFC, proliferation and migration of (pyramidal) PFC neurons, migration of GABAergic interneurons toward the PFC, axon guidance, target selection and synapse formation of PFC neurons, and PFC connectivity formation. Slowly, the view is emerging that some of these genes are identical to the susceptibility genes of neurodevelopmental disorders (Table 1). However, up to now only a few of the genes could be directly linked to one or more of the developmental events within the PFC as well as one or more of the four major neurodevelopmental disorders, that is, ID, ASDs, AD(H)D and/or schizophrenia.
Especially the availability of in utero electroporation-mediated gene transfer and other genetic approaches and hence the possibility to locally knock down or rescue particular genes will hopefully enable us to unravel the exact orchestration of brain areas such as those within the PFC in the near future. Such knowledge will assist in developing early intervention approaches by altering the susceptibility genes at a particular time and place, such that we deviate from the predetermined developmental path, even before the onset of the neurodevelopmental disorder(s) in question. Considering that individual susceptibility genes of neurodevelopmental disorders have often been found to be associated with multiple disorders, we can assume that several disorders share a common neurodevelopmental origin. It will be a challenge to dissect the individual genetic (and possibly even epigenetic) contributions to a disorder by using functional studies combined with behavioral tasks. For example, gene-environment interactions are crucial to distinguish between risk and vulnerability.
It is to be expected that in the coming years many more genes regulating developmental processes in the PFC and other brain structures will be linked to neurodevelopmental disorders and vice versa. Animal models, in which we can specifically alter gene expression in the PFC, can be instrumental for the understanding of the aetiopathological aspects of the disorder(s), as we can monitor the early disturbances that will eventually lead to defects in brain maturation and behavior. In order to move toward better and more preventive treatment of the neurodevelopmental disorders, bridges need to be built between disciplines such as combining genetic analyses of patients suffering from neurodevelopmental disorders with structural and functional brain imaging and in-depth molecular in vitro and in vivo approaches with cell and animal models. Exploring the molecular and cellular aspects during the progression of the disease process in animal models will clarify the pathological mechanisms, which in turn may provide clues to develop novel treatments for these disorders. The earlier during life and the more personalized the treatment strategies are applied, the better, alleviating symptoms at an early stage and reducing medical costs dramatically.
References
Patel V, Boyce N, Collins PY, Saxena S, Horton R . A renewed agenda for global mental health. Lancet 2011; 378: 1441–1442.
Belfer ML . Child and adolescent mental disorders: the magnitude of the problem across the globe. J Child Psychol Psychiatry 2008; 49: 226–236.
Mitchell SR, Reiss AL, Tatusko DH, Ikuta I, Kazmerski DB, Botti JA et al. Neuroanatomic alterations and social and communication deficits in monozygotic twins discordant for autism disorder. Am J Psychiatry 2009; 166: 917–925.
Shenton ME, Dickey CC, Frumin M, McCarley RW . A review of MRI findings in schizophrenia. Schizophr Res 2001; 49: 1–52.
Lesh TA, Niendam TA, Minzenberg MJ, Carter CS . Cognitive control deficits in schizophrenia:mechanisms and meaning. Neuropsychopharmacology 2011; 36: 316–338.
Casanova MF . Functional and anatomical aspects of prefrontal pathology in schizophrenia. Schizophrenia bulletin 1997; 23: 517–519.
Arnsten AF . Stress signalling pathways that impair prefrontal cortex structure and function. Nat Rev 2009; 10: 410–422.
Arnsten AF . Prefrontal cortical network connections: key site of vulnerability in stress and schizophrenia. Int J Dev Neurosci 29: 215–223.
Collins PY, Patel V, Joestl SS, March D, Insel TR, Daar AS et al. Grand challenges in global mental health. Nature 2011; 475: 27–30.
Kendler KS, Neale MC . Endophenotype: a conceptual analysis. Mol Psychiatry 2010; 15: 789–797.
Gamo NJ, Arnsten AF . Molecular modulation of prefrontal cortex: rational development of treatments for psychiatric disorders. Behav Neurosci 2012; 125: 282–296.
Lewis DA, Curley AA, Glausier JR, Volk DW . Cortical parvalbumin interneurons and cognitive dysfunction in schizophrenia. Trends Neurosci 2012; 35: 57–67.
Thompson-Schill SL, Bedny M, Goldberg RF . The frontal lobes and the regulation of mental activity. Curr Opin Neurobiol 2005; 15: 219–224.
Egner T, Hirsch J . Cognitive control mechanisms resolve conflict through cortical amplification of task-relevant information. Nat Neurosci 2005; 8: 1784–1790.
Etkin A, Egner T, Peraza DM, Kandel ER, Hirsch J . Resolving emotional conflict: a role for the rostral anterior cingulate cortex in modulating activity in the amygdala. Neuron 2006; 51: 871–882.
Miller EK . The prefrontal cortex and cognitive control. Nat Rev 2000; 1: 59–65.
Miller EK, Cohen JD . An integrative theory of prefrontal cortex function. Ann Rev Neurosci 2001; 24: 167–202.
Summerfield C, Egner T, Greene M, Koechlin E, Mangels J, Hirsch J . Predictive codes for forthcoming perception in the frontal cortex. Science 2006; 314: 1311–1314.
Duncan J . An adaptive coding model of neural function in prefrontal cortex. Nat Rev 2001; 2: 820–829.
Mansouri FA, Tanaka K, Buckley MJ . Conflict-induced behavioural adjustment: a clue to the executive functions of the prefrontal cortex. Nat Rev 2009; 10: 141–152.
Niwa M, Kamiya A, Murai R, Kubo K, Gruber AJ, Tomita K et al. Knockdown of DISC1 by in utero gene transfer disturbs postnatal dopaminergic maturation in the frontal cortex and leads to adult behavioral deficits. Neuron 2010; 65: 480–489.
Loos M, Mueller T, Gouwenberg Y, Wijnands R, van der Loo RJ, Birchmeier C et al. Neuregulin-3 in the mouse medial prefrontal cortex regulates impulsive action. Biol Psychiatry 2014; 76: 648–655.
Antonarakis SE, Lyle R, Dermitzakis ET, Reymond A, Deutsch S . Chromosome 21 and down syndrome: from genomics to pathophysiology. Nat Rev Genet. 2004; 5: 725–738.
Contestabile A, Benfenati F, Gasparini L . Communication breaks-down: from neurodevelopment defects to cognitive disabilities in Down syndrome. Prog Neurobiol 2010; 91: 1–22.
Gardiner K, Herault Y, Lott IT, Antonarakis SE, Reeves RH, Dierssen M . Down syndrome: from understanding the neurobiology to therapy. J Neurosci 2010; 30: 14943–14945.
van Bokhoven H . Genetic and epigenetic networks in intellectual disabilities. Annu Rev Genet 2011; 45: 81–104.
Chelly J, Mandel JL . Monogenic causes of X-linked mental retardation. Nat Rev Genet. 2001; 2: 669–680.
Kim M, Ceman S . Fragile X mental retardation protein: past, present and future. Curr Protein Pept Sci 2012; 13: 358–371.
Kleefstra T, Smidt M, Banning MJ, Oudakker AR, Van Esch H, de Brouwer AP et al. Disruption of the gene euchromatin histone methyl transferase1 (Eu-HMTase1) is associated with the 9q34 subtelomeric deletion syndrome. J Med Genet 2005; 42: 299–306.
Mercaldo V, Descalzi G, Zhuo M . Fragile X mental retardation protein in learning-related synaptic plasticity. Mol Cells 2009; 28: 501–507.
Cheng A, Haydar TF, Yarowsky PJ, Krueger BK . Concurrent generation of subplate and cortical plate neurons in developing trisomy 16 mouse cortex. Dev Neurosci 2004; 26: 255–265.
Chakrabarti L, Galdzicki Z, Haydar TF . Defects in embryonic neurogenesis and initial synapse formation in the forebrain of the Ts65Dn mouse model of Down syndrome. J Neurosci. 2007; 27: 11483–11495.
Willemsen MH, Vissers LE, Willemsen MA, van Bon BW, Kroes T, de Ligt J et al. Mutations in DYNC1H1 cause severe intellectual disability with neuronal migration defects. J Med Genet 49: 179–183.
Pilz DT, Matsumoto N, Minnerath S, Mills P, Gleeson JG, Allen KM et al. LIS1 and XLIS (DCX) mutations cause most classical lissencephaly, but different patterns of malformation. Hum Mol Genet 1998; 7: 2029–2037.
Rafalowska J, Dziewulska D, Podlecka A, Maslinska D . Early ontogenic disturbances in cell migration in mentally disabled adult. Clin Neuropathol 2001; 20: 13–18.
Penagarikano O, Abrahams BS, Herman EI, Winden KD, Gdalyahu A, Dong H et al. Absence of CNTNAP2 leads to epilepsy, neuronal migration abnormalities, and core autism-related deficits. Cell 2011; 147: 235–246.
Liu JS . Molecular genetics of neuronal migration disorders. Curr Neurol Neurosci Rep 11: 171–178.
Alarcon JM, Malleret G, Touzani K, Vronskaya S, Ishii S, Kandel ER et al. Chromatin acetylation, memory, and LTP are impaired in CBP+/− mice: a model for the cognitive deficit in Rubinstein-Taybi syndrome and its amelioration. Neuron 2004; 42: 947–959.
Nobs L, Baranek C, Nestel S, Kulik A, Kapfhammer J, Nitsch C et al. Stage-specific requirement for cyclin D1 in glial progenitor cells of the cerebral cortex. Glia 2014; 62: 829–839.
DiCicco-Bloom E, Lord C, Zwaigenbaum L, Courchesne E, Dager SR, Schmitz C et al. The developmental neurobiology of autism spectrum disorder. J Neurosci. 2006; 26: 6897–6906.
Grafodatskaya D, Chung B, Szatmari P, Weksberg R . Autism spectrum disorders and epigenetics. J Am Acad Child Adolesc Psychiatry 2010; 49: 794–809.
Amaral DG, Schumann CM, Nordahl CW . Neuroanatomy of autism. Trends Neurosci 2008; 31: 137–145.
Vorstman JA, Staal WG, van Daalen E, van Engeland H, Hochstenbach PF, Franke L . Identification of novel autism candidate regions through analysis of reported cytogenetic abnormalities associated with autism. Mol Psychiatry 2006; 11, 1 18–28.
Etkin A, Gyurak A, O'Hara R . A neurobiological approach to the cognitive deficits of psychiatric disorders. Dialogues Clin Neurosci 2013; 15: 419–429.
Bishop DV . Genes, cognition, and communication: insights from neurodevelopmental disorders. Ann NY Acad Sci. 2009; 1156: 1–18.
Folia V, Udden J, Forkstam C, Ingvar M, Hagoort P, Petersson KM . Implicit learning and dyslexia. Ann NY Acad Sci. 2008; 1145: 132–150.
Orban P, Lungu O, Doyon J . Motor sequence learning and developmental dyslexia. Ann NY Acad Sci. 2008; 1145: 151–172.
Pennington BF, Bishop DV . Relations among speech, language, and reading disorders. Annu Rev Psychol 2009; 60: 283–306.
Abrams DA, Lynch CJ, Cheng KM, Phillips J, Supekar K, Ryali S et al. Underconnectivity between voice-selective cortex and reward circuitry in children with autism. Proc Natl Acad Sci USA. 2013; 110: 12060–12065.
Vaccarino FM, Grigorenko EL, Smith KM, Stevens HE . Regulation of cerebral cortical size and neuron number by fibroblast growth factors: implications for autism. J Autism Dev Disord 2009; 39: 511–520.
Butler MG, Dasouki MJ, Zhou XP, Talebizadeh Z, Brown M, Takahashi TN et al. Subset of individuals with autism spectrum disorders and extreme macrocephaly associated with germline PTEN tumour suppressor gene mutations. J Med Genet 2005; 42: 318–321.
Courchesne E, Campbell K, Solso S . Brain growth across the life span in autism: age-specific changes in anatomical pathology. Brain Res. 2011; 1380: 138–145.
Courchesne E, Pierce K, Schumann CM, Redcay E, Buckwalter JA, Kennedy DP et al. Mapping early brain development in autism. Neuron 2007; 56: 399–413.
Schumann CM, Bloss CS, Barnes CC, Wideman GM, Carper RA, Akshoomoff N et al. Longitudinal magnetic resonance imaging study of cortical development through early childhood in autism. J Neurosci. 2010; 30: 4419–4427.
Fatemi SH, Halt AR, Stary JM, Kanodia R, Schulz SC, Realmuto GR . Glutamic acid decarboxylase 65 and 67 kDa proteins are reduced in autistic parietal and cerebellar cortices. Biol Psychiatry 2002; 52: 805–810.
Zhang ZW, Zak JD, Liu H . MeCP2 is required for normal development of GABAergic circuits in the thalamus. J Neurophysiol 2010; 103: 2470–2481.
Zikopoulos B, Barbas H . Changes in prefrontal axons may disrupt the network in autism. J Neurosci. 2010; 30: 14595–14609.
Zikopoulos B, Barbas H . Altered neural connectivity in excitatory and inhibitory cortical circuits in autism. Front Hum Neurosci 2013; 7: 609.
Medrihan L, Tantalaki E, Aramuni G, Sargsyan V, Dudanova I, Missler M et al. Early defects of GABAergic synapses in the brain stem of a MeCP2 mouse model of Rett syndrome. J Neurophysiol 2008; 99: 112–121.
Radyushkin K, Hammerschmidt K, Boretius S, Varoqueaux F, El-Kordi A, Ronnenberg A et al. Neuroligin-3-deficient mice: model of a monogenic heritable form of autism with an olfactory deficit. Genes Brain Behav 2009; 8: 416–425.
Santini E, Huynh TN, MacAskill AF, Carter AG, Pierre P, Ruggero D et al. Exaggerated translation causes synaptic and behavioural aberrations associated with autism. Nature 2013; 493: 411–415.
Testa-Silva G, Loebel A, Giugliano M, de Kock CP, Mansvelder HD, Meredith RM . Hyperconnectivity and slow synapses during early development of medial prefrontal cortex in a mouse model for mental retardation and autism. Cereb Cortex. 2012; 22: 1333–1342.
Morgan JT, Chana G, Pardo CA, Achim C, Semendeferi K, Buckwalter J et al. Microglial activation and increased microglial density observed in the dorsolateral prefrontal cortex in autism. Biol Psychiatry 2010; 68: 368–376.
Morgan JT, Chana G, Abramson I, Semendeferi K, Courchesne E, Everall IP . Abnormal microglial-neuronal spatial organization in the dorsolateral prefrontal cortex in autism. Brain Res. 2012; 1456: 72–81.
Biederman J . Attention-deficit/hyperactivity disorder: a selective overview. Biol Psychiatry 2005; 57: 1215–1220.
Liston C, Malter Cohen M, Teslovich T, Levenson D, Casey BJ . Atypical prefrontal connectivity in attention-deficit/hyperactivity disorder: pathway to disease or pathological end point? Biol Psychiatry 2011; 69: 1168–1177.
Brennan AR, Arnsten AF . Neuronal mechanisms underlying attention deficit hyperactivity disorder: the influence of arousal on prefrontal cortical function. Ann NY Acad Sci. 2008; 1129: 236–245.
Shaw P, Eckstrand K, Sharp W, Blumenthal J, Lerch JP, Greenstein D et al. Attention- deficit/hyperactivity disorder is characterized by a delay in cortical maturation. Proc Natl Acad Sci USA. 2007; 104: 19649–19654.
Maltezos S, Horder J, Coghlan S, Skirrow C, O'Gorman R, Lavender TJ et al. Glutamate/glutamine and neuronal integrity in adults with ADHD: a proton MRS study. Transl Psychiatry 2014; 4: e373.
D'Agati E, Casarelli L, Pitzianti MB, Pasini A . Overflow movements and white matter abnormalities in ADHD. Prog Neuropsychopharmacol Biol Psychiatry 2010; 34: 441–445.
Xia S, Li X, Kimball AE, Kelly MS, Lesser I, Branch C . Thalamic shape and connectivity abnormalities in children with attention-deficit/hyperactivity disorder. Psychiatry Res. 2012; 204: 161–167.
Hart H, Marquand AF, Smith A, Cubillo A, Simmons A, Brammer M et al. Predictive neurofunctional markers of attention-deficit/hyperactivity disorder based on pattern classification of temporal processing. J Am Acad Child Adolesc Psychiatry 2014; 53: 569–78, e1.
Arnsten AF . Fundamentals of attention-deficit/hyperactivity disorder: circuits and pathways. J Clin Psychiatry 2006; 67 (Suppl 8): 7–12.
Rommelse NN, Geurts HM, Franke B, Buitelaar JK, Hartman CA . A review on cognitive and brain endophenotypes that may be common in autism spectrum disorder and attention- deficit/hyperactivity disorder and facilitate the search for pleiotropic genes. Neurosci Biobehav Rev 2011; 35: 1363–1396.
Leo D, Sorrentino E, Volpicelli F, Eyman M, Greco D, Viggiano D et al. Altered midbrain dopaminergic neurotransmission during development in an animal model of ADHD. Neurosci Biobehav Rev 2003; 27: 661–669.
Miller EM, Pomerleau F, Huettl P, Gerhardt GA, Glaser PE . Aberrant glutamate signaling in the prefrontal cortex and striatum of the spontaneously hypertensive rat model of attention- deficit/hyperactivity disorder. Psychopharmacology 2014; 231: 3019–3029.
Fossella JA, Sommer T, Fan J, Pfaff D, Posner MI . Synaptogenesis and heritable aspects of executive attention. Ment Retard Dev Disabil Res Rev 2003; 9: 178–183.
Kenar AN, Ay OI, Herken H, Erdal ME . Association of VAMP-2 and Syntaxin 1A genes with adult attention deficit hyperactivity disorder. Psychiatry Investig 2014; 11: 76–83.
Hawi Z, Matthews N, Wagner J, Wallace RH, Butler TJ, Vance A et al. DNA variation in the SNAP25 gene confers risk to ADHD and is associated with reduced expression in prefrontal cortex. PloS One 2013; 8: e60274.
McCarthy SE, McCombie WR, Corvin A . Unlocking the treasure trove: from genes to schizophrenia biology. Schizophr Bull 2014; 40: 492–496.
Lewis DA, Cruz D, Eggan S, Erickson S . Postnatal development of prefrontal inhibitory circuits and the pathophysiology of cognitive dysfunction in schizophrenia. Ann NY Acad Sci. 2004; 1021: 64–76.
Connor CM, Guo Y, Akbarian S . Cingulate white matter neurons in schizophrenia and bipolar disorder. Biol Psychiatry 2009; 66: 486–493.
Beneyto M, Lewis DA . Insights into the neurodevelopmental origin of schizophrenia from postmortem studies of prefrontal cortical circuitry. Int J Dev Neurosci. 2011; 29: 295–304.
Arnsten AF . Prefrontal cortical network connections: key site of vulnerability in stress and schizophrenia. Int J Dev Neurosci. 2011; 29: 215–223.
Zierhut KC, Schulte-Kemna A, Kaufmann J, Steiner J, Bogerts B, Schiltz K . Distinct structural alterations independently contributing to working memory deficits and symptomatology in paranoid schizophrenia. Cortex 2012; 49: 1063–1072.
Arnold SE, Talbot K, Hahn CG . Neurodevelopment, neuroplasticity, and new genes for schizophrenia. Prog Brain Res 2005; 147: 319–345.
Bernstein HG, Smalla KH, Durrschmidt D, Keilhoff G, Dobrowolny H, Steiner J et al. Increased density of prohibitin-immunoreactive oligodendrocytes in the dorsolateral prefrontal white matter of subjects with schizophrenia suggests extraneuronal roles for the protein in the disease. Neuromolecular Med. 2012; 14: 270–280.
Glantz LA, Lewis DA . Decreased dendritic spine density on prefrontal cortical pyramidal neurons in schizophrenia. Arch Gen Psychiatry 2000; 57: 65–73.
Bennett MR . Schizophrenia: susceptibility genes, dendritic-spine pathology and gray matter loss. Prog Neurobiol 2011; 95: 275–300.
Glausier JR, Fish KN, Lewis DA . Altered parvalbumin basket cell inputs in the dorsolateral prefrontal cortex of schizophrenia subjects. Mol Psychiatry 2014; 19: 30–36.
Glausier JR, Lewis DA . Dendritic spine pathology in schizophrenia. Neuroscience 2013; 251: 90–107.
Carlsson A . The neurochemical circuitry of schizophrenia. Pharmacopsychiatry 2006; 39: S10–S14.
Seamans JK, Yang CR . The principal features and mechanisms of dopamine modulation in the prefrontal cortex. Prog Neurobiol 2004; 74: 1–58.
Gonzalez-Burgos G, Hashimoto T, Lewis DA . Alterations of cortical GABA neurons and network oscillations in schizophrenia. Curr Psychiatry Rep 2010; 12: 335–344.
Lewis DA, Gonzalez-Burgos G . Neuroplasticity of neocortical circuits in schizophrenia. Neuropsychopharmacology 2008; 33: 141–165.
Volk DW, Lewis DA . Prefrontal cortical circuits in schizophrenia. Curr Top Behav Neurosci 2010; 4: 485–508.
Rolls ET, Loh M, Deco G, Winterer G . Computational models of schizophrenia and dopamine modulation in the prefrontal cortex. Nat Rev 2008; 9: 696–709.
Chen L, Perez SM, Lodge DJ . An augmented dopamine system function is present prior to puberty in the methylazoxymethanol acetate rodent model of schizophrenia. Dev Neurobiol. 2014; 74: 907–917.
Deserno L, Sterzer P, Wustenberg T, Heinz A, Schlagenhauf F . Reduced prefrontal-parietal effective connectivity and working memory deficits in schizophrenia. J Neurosci. 2012; 32: 12–20.
Elston GN . Cortex, cognition and the cell: new insights into the pyramidal neuron and prefrontal function. Cereb Cortex. 2003; 13: 1124–1138.
Ongur D, Price JL . The organization of networks within the orbital and medial prefrontal cortex of rats, monkeys and humans. Cereb Cortex. 2000; 10: 206–219.
Kellendonk C, Simpson EH, Kandel ER . Modeling cognitive endophenotypes of schizophrenia in mice. Trends Neurosci 2009; 32: 347–358.
Jaaro-Peled H . Gene models of schizophrenia: DISC1 mouse models. Prog Brain Res 2009; 179: 75–86.
van Loo KM, Martens GJ . Genetic and environmental factors in complex neurodevelopmental disorders. Curr Genomics 2007; 8: 429–444.
Coolen MW, Van Loo KM, Van Bakel NN, Pulford DJ, Serneels L, De Strooper B et al. Gene dosage effect on gamma-secretase component Aph-1b in a rat model for neurodevelopmental disorders. Neuron 2005; 45: 497–503.
Van Schijndel JE, Van Zweeden M, Van Loo KM, Martens GJ . Gene expression profiling in brain regions of a rat model displaying schizophrenia-related features. Behav Brain Res 2010; 207: 476–479.
van der Elst MC, Roubos EW, Ellenbroek BA, Veening JG, Cools AR . Apomorphine-susceptible rats and apomorphine-unsusceptible rats differ in the tyrosine hydroxylase-immunoreactive network in the nucleus accumbens core and shell. Exp Brain Res 2005; 160: 418–423.
Moron JA, Brockington A, Wise RA, Rocha BA, Hope BT . Dopamine uptake through the norepinephrine transporter in brain regions with low levels of the dopamine transporter: evidence from knock-out mouse lines. J Neurosci. 2002; 22: 389–395.
Roullet FI, Crawley JN . Mouse models of autism: testing hypotheses about molecular mechanisms. Curr Top Behav Neurosci 2011; 7: 187–212.
Moy SS, Nadler JJ . Advances in behavioral genetics: mouse models of autism. Mol Psychiatry 2008; 13: 4–26.
Desbonnet L, Waddington JL, Tuathaigh CM . Mice mutant for genes associated with schizophrenia: common phenotype or distinct endophenotypes? Behav Brain Res 2009; 204: 258–273.
Johnstone M, Thomson PA, Hall J, McIntosh AM, Lawrie SM, Porteous DJ . DISC1 in schizophrenia: genetic mouse models and human genomic imaging. Schizophr Bull 2011; 37: 14–20.
Sawa A . Genetic animal models for schizophrenia: advantages and limitations of genetic manipulation in drosophila, zebrafish, rodents, and primates. Prog Brain Res 2009; 179: 3–6.
Chen J, Lipska BK, Weinberger DR . Genetic mouse models of schizophrenia: from hypothesis-based to susceptibility gene-based models. Biol Psychiatry 2006; 59: 1180–1188.
Baek ST, Kerjan G, Bielas SL, Lee JE, Fenstermaker AG, Novarino G et al. Off-target effect of doublecortin family shRNA on neuronal migration associated with endogenous microRNA dysregulation. Neuron 2014; 82: 1255–1262.
Kolk SM, de Mooij-Malsen AJ, Martens GJ . Spatiotemporal molecular approach of in utero electroporation to functionally decipher endophenotypes in neurodevelopmental disorders. Front Mol Neurosci 2011; 4: 37.
Bissonette GB, Powell EM . Reversal learning and attentional set-shifting in mice. Neuropharmacology 2011; 62: 1168–1174.
Bissonette GB, Martins GJ, Franz TM, Harper ES, Schoenbaum G, Powell EM . Double dissociation of the effects of medial and orbital prefrontal cortical lesions on attentional and affective shifts in mice. J Neurosci. 2008; 28: 11124–11130.
Brigman JL, Rothblat LA . Stimulus specific deficit on visual reversal learning after lesions of medial prefrontal cortex in the mouse. Behav Brain Res 2008; 187: 405–410.
Kellendonk C, Simpson EH, Polan HJ, Malleret G, Vronskaya S, Winiger V et al. Transient and selective overexpression of dopamine D2 receptors in the striatum causes persistent abnormalities in prefrontal cortex functioning. Neuron 2006; 49: 603–615.
Ben Abdallah NM, Fuss J, Trusel M, Galsworthy MJ, Bobsin K, Colacicco G et al. The puzzle box as a simple and efficient behavioral test for exploring impairments of general cognition and executive functions in mouse models of schizophrenia. Exp Neurol 2011; 227: 42–52.
Chudasama Y . Animal models of prefrontal-executive function. Behav Neurosci 2011; 125: 327–343.
Kolb B, Mychasiuk R, Muhammad A, Li Y, Frost DO, Gibb R . Experience and the developing prefrontal cortex. Proc Natl Acad Sci USA. 2012; 109: 17186–17193.
Vieira C, Pombero A, Garcia-Lopez R, Gimeno L, Echevarria D, Martinez S . Molecular mechanisms controlling brain development: an overview of neuroepithelial secondary organizers. Int J Dev Biol 2010; 54: 7–20.
Puelles L, Rubenstein JL . Forebrain gene expression domains and the evolving prosomeric model. Trends Neurosci 2003; 26: 469–476.
Andersson E, Tryggvason U, Deng Q, Friling S, Alekseenko Z, Robert B et al. Identification of intrinsic determinants of midbrain dopamine neurons. Cell 2006; 124: 393–405.
Pombero A, Martinez S . Telencephalic morphogenesis during the process of neurulation: an experimental study using quail-chick chimeras. J Comp Neurol 2009; 512: 784–797.
Rubenstein JL, Martinez S, Shimamura K, Puelles L . The embryonic vertebrate forebrain: the prosomeric model. Science (New York, NY 1994; 266: 578–580.
Borello U, Pierani A . Patterning the cerebral cortex: traveling with morphogens. Curr Opin Genet Dev 2010; 20: 408–415.
Lander AD . Morpheus unbound: reimagining the morphogen gradient. Cell 2007; 128: 245–256.
O'Leary DD, Chou SJ, Sahara S . Area patterning of the mammalian cortex. Neuron 2007; 56: 252–269.
Fukuchi-Shimogori T, Grove EA . Neocortex patterning by the secreted signaling molecule FGF8. Science (New York, NY 2001; 294: 1071–1074.
Rubenstein JL . Research Review: Development of the cerebral cortex: implications for neurodevelopmental disorders. J Child Psychol Psychiatry 2010; 52: 339–355.
Hebert JM, Hayhurst M, Marks ME, Kulessa H, Hogan BL, McConnell SK . BMP ligands act redundantly to pattern the dorsal telencephalic midline. Genesis 2003; 35: 214–219.
Fernandes M, Gutin G, Alcorn H, McConnell SK, Hebert JM . Mutations in the BMP pathway in mice support the existence of two molecular classes of holoprosencephaly. Development (Cambridge, England) 2007; 134: 3789–3794.
Hebert JM, Mishina Y, McConnell SK . BMP signaling is required locally to pattern the dorsal telencephalic midline. Neuron 2002; 35: 1029–1041.
Rash BG, Grove EA . Patterning the dorsal telencephalon: a role for sonic hedgehog? J Neurosci 2007; 27: 11595–11603.
Kuschel S, Ruther U, Theil T . A disrupted balance between Bmp/Wnt and Fgf signaling underlies the ventralization of the Gli3 mutant telencephalon. Dev Biol 2003; 260: 484–495.
Ohkubo Y, Chiang C, Rubenstein JL . Coordifnate regulation and synergistic actions of BMP4, SHH and FGF8 in the rostral prosencephalon regulate morphogenesis of the telencephalic and optic vesicles. Neuroscience 2002; 111: 1–17.
Blaess S, Stephen D, Joyner AL . Gli3 coordinates three-dimensional patterning and growth of the tectum and cerebellum by integrating Shh and Fgf8 signaling. Development (Cambridge, England) 2008; 135: 2093–2103.
Cholfin JA, Rubenstein JL . Frontal cortex subdivision patterning is coordinately regulated by Fgf8, Fgf17, and Emx2. J Comp Neurol 2008; 509: 144–155.
Scearce-Levie K, Roberson ED, Gerstein H, Cholfin JA, Mandiyan VS, Shah NM et al. Abnormal social behaviors in mice lacking Fgf17. Genes Brain Behav 2008; 7: 344–354.
Shimogori T, Banuchi V, Ng HY, Strauss JB, Grove EA . Embryonic signaling centers expressing BMP, WNT and FGF proteins interact to pattern the cerebral cortex. Development (Cambridge, England) 2004; 131: 5639–5647.
Shin DM, Korada S, Raballo R, Shashikant CS, Simeone A, Taylor JR et al. Loss of glutamatergic pyramidal neurons in frontal and temporal cortex resulting from attenuation of FGFR1 signaling is associated with spontaneous hyperactivity in mice. J Neurosci. 2004; 24: 2247–2258.
Thomson RE, Kind PC, Graham NA, Etherson ML, Kennedy J, Fernandes AC et al. Fgf receptor 3 activation promotes selective growth and expansion of occipitotemporal cortex. Neural Dev 2009; 4: 4.
Thomson RE, Pellicano F, Iwata T . Fibroblast growth factor receptor 3 kinase domain mutation increases cortical progenitor proliferation via mitogen-activated protein kinase activation. J Neurochem 2007; 100: 1565–1578.
Stevens HE, Smith KM, Maragnoli ME, Fagel D, Borok E, Shanabrough M et al. Fgfr2 is required for the development of the miedial prefrontal cortex and its connections with limbic circuits. J Neurosci. 2010; 30: 5590–5602.
Muller Smith K, Fagel DM, Stevens HE, Rabenstein RL, Maragnoli ME, Ohkubo Y et al. Deficiency in inhibitory cortical interneurons associates with hyperactivity in fibroblast growth factor receptor 1 mutant mice. Biol Psychiatry 2008; 63: 953–962.
Inglis-Broadgate SL, Thomson RE, Pellicano F, Tartaglia MA, Pontikis CC, Cooper JD et al. FGFR3 regulates brain size by controlling progenitor cell proliferation and apoptosis during embryonic development. Dev Biol 2005; 279: 73–85.
Hoch RV, Rubenstein JL, Pleasure S . Genes and signaling events that establish regional patterning of the mammalian forebrain. Semin Cell Dev Biol 2009; 20: 378–386.
Sur M, Rubenstein JL . Patterning and plasticity of the cerebral cortex. Science (New York, NY 2005; 310: 805–810.
Mason I . Initiation to end point: the multiple roles of fibroblast growth factors in neural development. Nat Rev 2007; 8: 583–596.
Schuurmans C, Guillemot F . Molecular mechanisms underlying cell fate specification in the developing telencephalon. Curr Opin Neurobiol 2002; 12: 26–34.
Jessell TM . Neuronal specification in the spinal cord: inductive signals and transcriptional codes. Nat Rev Genet 2000; 1: 20–29.
Goridis C, Rohrer H . Specification of catecholaminergic and serotonergic neurons. Nat Rev 2002; 3: 531–541.
Hasegawa H, Ashigaki S, Takamatsu M, Suzuki-Migishima R, Ohbayashi N, Itoh N et al. Laminar patterning in the developing neocortex by temporally coordinated fibroblast growth factor signaling. J Neurosci 2004; 24: 8711–8719.
Chou SJ, Perez-Garcia CG, Kroll TT, O'Leary DD . Lhx2 specifies regional fate in Emx1 lineage of telencephalic progenitors generating cerebral cortex. Nat Neurosci 2009; 12: 1381–1389.
Mangale VS, Hirokawa KE, Satyaki PR, Gokulchandran N, Chikbire S, Subramanian L et al. Lhx2 selector activity specifies cortical identity and suppresses hippocampal organizer fate. Science (New York, NY 2008; 319: 304–309.
Polleux F, Ince-Dunn G, Ghosh A . Transcriptional regulation of vertebrate axon guidance and synapse formation. Nat Rev 2007; 8: 331–340.
Flames N, Pla R, Gelman DM, Rubenstein JL, Puelles L, Marin O . Delineation of multiple subpallial progenitor domains by the combinatorial expression of transcriptional codes. J Neurosci 2007; 27: 9682–9695.
Uchida S, Hara K, Kobayashi A, Funato H, Hobara T, Otsuki K et al. Early life stress enhances behavioral vulnerability to stress through the activation of REST4-mediated gene transcription in the medial prefrontal cortex of rodents. J Neurosci 2010; 30: 15007–15018.
Rojas P, Joodmardi E, Hong Y, Perlmann T, Ogren SO . Adult mice with reduced Nurr1 expression: an animal model for schizophrenia. Mol Psychiatry 2007; 12: 756–766.
Johnson MB, Kawasawa YI, Mason CE, Krsnik Z, Coppola G, Bogdanovic D et al. Functional and evolutionary insights into human brain development through global transcriptome analysis. Neuron 2009; 62: 494–509.
Yang S, Wang K, Valladares O, Hannenhalli S, Bucan M . Genome-wide expression profiling and bioinformatics analysis of diurnally regulated genes in the mouse prefrontal cortex. Genome Biol 2007; 8: R247.
Erraji-Benchekroun L, Underwood MD, Arango V, Galfalvy H, Pavlidis P, Smyrniotopoulos P et al. Molecular aging in human prefrontal cortex is selective and continuous throughout adult life. Biol Psychiatry 2005; 57: 549–558.
Zhang YE, Landback P, Vibranovski MD, Long M . Accelerated recruitment of new brain development genes into the human genome. PLoS Biol 2011; 9: e1001179.
Kriegstein AR, Noctor SC . Patterns of neuronal migration in the embryonic cortex. Trends Neurosci 2004; 27: 392–399.
Noctor SC, Flint AC, Weissman TA, Dammerman RS, Kriegstein AR . Neurons derived from radial glial cells establish radial units in neocortex. Nature 2001; 409: 714–720.
Storm EE, Garel S, Borello U, Hebert JM, Martinez S, McConnell SK et al. Dose-dependent functions of Fgf8 in regulating telencephalic patterning centers. Development (Cambridge, England) 2006; 133: 1831–1844.
Garel S, Huffman KJ, Rubenstein JL . Molecular regionalization of the neocortex is disrupted in Fgf8 hypomorphic mutants. Development (Cambridge, England) 2003; 130: 1903–1914.
Raballo R, Rhee J, Lyn-Cook R, Leckman JF, Schwartz ML, Vaccarino FM . Basic fibroblast growth factor (Fgf2) is necessary for cell proliferation and neurogenesis in the developing cerebral cortex. J Neurosci 2000; 20: 5012–5023.
Sessa A, Mao CA, Hadjantonakis AK, Klein WH, Broccoli V . Tbr2 directs conversion of radial glia into basal precursors and guides neuronal amplification by indirect neurogenesis in the developing neocortex. Neuron 2008; 60: 56–69.
Sessa A, Mao CA, Colasante G, Nini A, Klein WH, Broccoli V . Tbr2-positive intermediate (basal) neuronal progenitors safeguard cerebral cortex expansion by controlling amplification of pallial glutamatergic neurons and attraction of subpallial GABAergic interneurons. Genes Dev 2010; 24: 1816–1826.
Arnold SJ, Huang GJ, Cheung AF, Era T, Nishikawa S, Bikoff EK et al. The T-box transcription factor Eomes/Tbr2 regulates neurogenesis in the cortical subventricular zone. Genes Dev 2008; 22: 2479–2484.
Sweeney NT, Tierney H, Feldheim DA . Tbr2 is required to generate a neural circuit mediating the pupillary light reflex. J Neurosci 2014; 34: 5447–5453.
Kolk SM, Gunput RA, Tran TS, van den Heuvel DM, Prasad AA, Hellemons AJ et al. Semaphorin 3F is a bifunctional guidance cue for dopaminergic axons and controls their fasciculation, channeling, rostral growth, and intracortical targeting. J Neurosci 2009; 29: 12542–12557.
Kinast K, Peeters D, Kolk SM, Schubert D, Homberg JR . Genetic and pharmacological manipulations of the serotonergic system in early life: neurodevelopmental underpinnings of autism-related behavior. Front Cell Neurosci 2013; 7: 72.
Popolo M, McCarthy DM, Bhide PG . Influence of dopamine on precursor cell proliferation and differentiation in the embryonic mouse telencephalon. Dev Neurosci 2004; 26: 229–244.
Kriegstein A, Noctor S, Martinez-Cerdeno V . Patterns of neural stem and progenitor cell division may underlie evolutionary cortical expansion. Nat Rev 2006; 7: 883–890.
Ayala R, Shu T, Tsai LH . Trekking across the brain: the journey of neuronal migration. Cell 2007; 128: 29–43.
Rakic P . The radial edifice of cortical architecture: from neuronal silhouettes to genetic engineering. Brain Res Rev 2007; 55: 204–219.
Knuesel I . Reelin-mediated signaling in neuropsychiatric and neurodegenerative diseases. Prog Neurobiol 2010; 91: 257–274.
Gaiano N . Strange bedfellows: Reelin and Notch signaling interact to regulate cell migration in the developing neocortex. Neuron 2008; 60: 189–191.
Lui JH, Hansen DV, Kriegstein AR . Development and evolution of the human neocortex. Cell 2011; 146: 18–36.
LaMonica BE, Lui JH, Wang X, Kriegstein AR . OSVZ progenitors in the human cortex: an updated perspective on neurodevelopmental disease. Curr Opin Neurobiol 2012; 22: 747–753.
Gertz CC, Lui JH, LaMonica BE, Wang X, Kriegstein AR . Diverse behaviors of outer radial glia in developing ferret and human cortex. J Neurosci 2014; 34: 2559–2570.
Miyoshi G, Butt SJ, Takebayashi H, Fishell G . Physiologically distinct temporal cohorts of cortical interneurons arise from telencephalic Olig2-expressing precursors. J Neurosci 2007; 27: 7786–7798.
Southwell DG, Nicholas CR, Basbaum AI, Stryker MP, Kriegstein AR, Rubenstein JL et al. Interneurons from embryonic development to cell-based therapy. Science (New York, NY 2014; 344: 1240622.
Cobos I, Long JE, Thwin MT, Rubenstein JL . Cellular patterns of transcription factor expression in developing cortical interneurons. Cereb Cortex 2006; 16: i82–i88.
Le Magueresse C, Monyer H . GABAergic interneurons shape the functional maturation of the cortex. Neuron 2013; 77: 388–405.
Tessier-Lavigne M, Goodman CS . The molecular biology of axon guidance. Science (New York, NY 1996; 274: 1123–1133.
Dickson BJ . Molecular mechanisms of axon guidance. Science (New York, NY 2002; 298: 1959–1964.
O'Donnell M, Chance RK, Bashaw GJ . Axon growth and guidance: receptor regulation and signal transduction. Annu Rev Neurosci 2009; 32: 383–412.
Dudanova I, Klein R . Integration of guidance cues: parallel signaling and crosstalk. Trends Neurosci 2013; 36: 295–304.
Kiryushko D, Berezin V, Bock E . Regulators of neurite outgrowth: role of cell adhesion molecules. Ann NY Acad Sci 2004; 1014: 140–154.
Stoya G, Redies C, Schmid-Hertel N . Inversion of layer-specific cadherin expression profiles and maintenance of cytoarchitectonic areas in the allocortex of the reeler mutant mouse. J Comp Neurol 2014.
Lai KO, Ip NY . Synapse development and plasticity: roles of ephrin/Eph receptor signaling. Curr Opin Neurobiol 2009; 19: 275–283.
Tessier CR, Broadie K . Activity-dependent modulation of neural circuit synaptic connectivity. Front Mol Neurosci 2009; 2: 8.
Vanderhaeghen P, Cheng HJ . Guidance molecules in axon pruning and cell death. Cold Spring Harbor Perspect Biol 2010; 2: a001859.
Petanjek Z, Judas M, Kostovic I, Uylings HB . Lifespan alterations of basal dendritic trees of pyramidal neurons in the human prefrontal cortex: a layer-specific pattern. Cereb Cortex 2008; 18: 915–929.
de Almeida J, Jourdan I, Murer MG, Belforte JE . Refinement of neuronal synchronization with gamma oscillations in the medial prefrontal cortex after adolescence. PloS One 2013; 8: e62978.
Yang JM, Zhang J, Yu YQ, Duan S, Li XM . Postnatal development of 2 microcircuits involving fast-spiking interneurons in the mouse prefrontal cortex. Cereb Cortex 2014; 24: 98–109.
Hoover WB, Vertes RP . Anatomical analysis of afferent projections to the medial prefrontal cortex in the rat. Brain Struct Funct 2007; 212: 149–179.
George O, Koob GF . Individual differences in prefrontal cortex function and the transition from drug use to drug dependence. Neurosci Biobehav Rev 2010; 35: 232–247.
Lisboa SF, Stecchini MF, Correa FM, Guimaraes FS, Resstel LB . Different role of the ventral medial prefrontal cortex on modulation of innate and associative learned fear. Neuroscience 2010; 171: 760–768.
Diamond A . Biological and social influences on cognitive control processes dependent on prefrontal cortex. Prog Brain Res 2011; 189: 319–339.
Thompson BM, Baratta MV, Biedenkapp JC, Rudy JW, Watkins LR, Maier SF . Activation of the infralimbic cortex in a fear context enhances extinction learning. Learn Mem 2010; 17: 591–599.
Laroche S, Davis S, Jay TM . Plasticity at hippocampal to prefrontal cortex synapses: dual roles in working memory and consolidation. Hippocampus 2000; 10: 438–446.
Little JP, Carter AG . Subcellular synaptic connectivity of layer 2 pyramidal neurons in the medial prefrontal cortex. J Neurosci 2012; 32: 12808–12819.
Golmayo L, Nunez A, Zaborszky L . Electrophysiological evidence for the existence of a posterior cortical-prefrontal-basal forebrain circuitry in modulating sensory responses in visual and somatosensory rat cortical areas. Neuroscience 2003; 119: 597–609.
Henny P, Jones BE . Projections from basal forebrain to prefrontal cortex comprise cholinergic, GABAergic and glutamatergic inputs to pyramidal cells or interneurons. Eur J Neurosci 2008; 27: 654–670.
Hauber W . Dopamine release in the prefrontal cortex and striatum: temporal and behavioural aspects. Pharmacopsychiatry 43: S32–S41.
van Schouwenburg M, Aarts E, Cools R . Dopaminergic modulation of cognitive control:distinct roles for the prefrontal cortex and the basal ganglia. Curr Pharm Des 2010; 16: 2026–2032.
Fitzgerald PJ . A neurochemical yin and yang: does serotonin activate and norepinephrine deactivate the prefrontal cortex? Psychopharmacology 2011; 213: 171–182.
Witteveen JS, Middelman A, van Hulten JA, Martens GJ, Homberg JR, Kolk SM . Lack of serotonin reuptake during brain development alters rostral raphe-prefrontal network formation. Front Cell Neurosci 2013; 7: 143.
Goto Y, O'Donnell P . Altered prefrontal cortex-nucleus accumbens information processing in a developmental animal model of schizophrenia. Ann NY Acad Sci 2003; 1003: 398–401.
Goto Y, Grace AA . Limbic and cortical information processing in the nucleus accumbens. Trends Neurosci 2008; 31: 552–558.
Sarter M, Hasselmo ME, Bruno JP, Givens B . Unraveling the attentional functions of cortical cholinergic inputs: interactions between signal-driven and cognitive modulation of signal detection. Brain Res 2005; 48: 98–111.
Del Arco A, Mora F . Neurotransmitters and prefrontal cortex-limbic system interactions: implications for plasticity and psychiatric disorders. J Neural Transm 2009; 116: 941–952.
Zhang ZW, Burke MW, Calakos N, Beaulieu JM, Vaucher E . Confocal analysis of cholinergic and dopaminergic inputs onto pyramidal cells in the prefrontal cortex of rodents. Front Neuroanat 2010; 4: 21.
Otani S, Daniel H, Roisin MP, Crepel F . Dopaminergic modulation of long-term synaptic plasticity in rat prefrontal neurons. Cereb Cortex 2003; 13: 1251–1256.
Gabbott P, Headlam A, Busby S . Morphological evidence that CA1 hippocampal afferents monosynaptically innervate PV-containing neurons and NADPH-diaphorase reactive cells in the medial prefrontal cortex (Areas 25/32) of the rat. Brain Res 2002; 946: 314–322.
Gabbott PL, Warner TA, Busby SJ . Amygdala input monosynaptically innervates parvalbumin immunoreactive local circuit neurons in rat medial prefrontal cortex. Neuroscience 2006; 139: 1039–1048.
Rotaru DC, Barrionuevo G, Sesack SR . Mediodorsal thalamic afferents to layer III of the rat prefrontal cortex: synaptic relationships to subclasses of interneurons. J Comp Neurol 2005; 490: 220–238.
Little JP, Carter AG . Synaptic mechanisms underlying strong reciprocal connectivity between the medial prefrontal cortex and basolateral amygdala. J Neurosci 2013; 33: 15333–15342.
Ernst M, Fudge JL . A developmental neurobiological model of motivated behavior: anatomy, connectivity and ontogeny of the triadic nodes. Neurosci Biobehav Reviews 2009; 33: 367–382.
Ernst M, Pine DS, Hardin M . Triadic model of the neurobiology of motivated behavior in adolescence. Psychol Med 2006; 36: 299–312.
Senn V, Wolff SB, Herry C, Grenier F, Ehrlich I, Grundemann J et al. Long-range connectivity defines behavioral specificity of amygdala neurons. Neuron 2014; 81: 428–437.
Heidbreder CA, Groenewegen HJ . The medial prefrontal cortex in the rat: evidence for a dorso-ventral distinction based upon functional and anatomical characteristics. Neurosci Biobehav Rev 2003; 27: 555–579.
Gabbott PL, Warner TA, Jays PR, Salway P, Busby SJ . Prefrontal cortex in the rat: projections to subcortical autonomic, motor, and limbic centers. J Comp Neurol 2005; 492: 145–177.
Dembrow NC, Chitwood RA, Johnston D . Projection-specific neuromodulation of medial prefrontal cortex neurons. J Neurosci 2010; 30: 16922–16937.
Ding DC, Gabbott PL, Totterdell S . Differences in the laminar origin of projections from the medial prefrontal cortex to the nucleus accumbens shell and core regions in the rat. Brain Res 2001; 917: 81–89.
Cholfin JA, Rubenstein JL . Genetic regulation of prefrontal cortex development and function. Novartis Found Symp. 2007; 288: 165–173, discussion 73–7, 276–81.
Schell-Apacik C, Rivero M, Knepper JL, Roessler E, Muenke M, Ming JE . SONIC HEDGEHOG mutations causing human holoprosencephaly impair neural patterning activity. Hum Genet. 2003; 113: 170–177.
Al-Ayadhi LY . Relationship between Sonic hedgehog protein, brain-derived neurotrophic factor and oxidative stress in autism spectrum disorders. Neurochem Res. 2012; 37: 394–400.
Heussler HS, Suri M, Young ID, Muenke M . Extreme variability of expression of a Sonic Hedgehog mutation: attention difficulties and holoprosencephaly. Arch Dis Child. 2002; 86: 293–296.
Raballo R, Rhee J, Lyn-Cook R, Leckman JF, Schwartz ML, Vaccarino FM . Basic fibroblast growth factor (Fgf2) is necessary for cell proliferation and neurogenesis in the developing cerebral cortex. J Neurosci. 2000; 20: 5012–5023.
Terwisscha van Scheltinga AF, Bakker SC, Kahn RS, Kas MJ . Fibroblast growth factors in neurodevelopment and psychopathology. Neuroscientist 2013; 19: 479–494.
Hashimoto K, Shimizu E, Komatsu N, Nakazato M, Okamura N, Watanabe H et al. Increased levels of serum basic fibroblast growth factor in schizophrenia. Psychiatry Res. 2003; 120: 211–218.
Simonis N, Migeotte I, Lambert N, Perazzolo C, de Silva DC, Dimitrov B et al. FGFR1 mutations cause Hartsfield syndrome, the unique association of holoprosencephaly and ectrodactyly. J Med Genet 2013; 50: 585–592.
Gaughran F, Payne J, Sedgwick PM, Cotter D, Berry M . Hippocampal FGF-2 and FGFR1 mRNA expression in major depression, schizophrenia and bipolar disorder. Brain Res Bull 2006; 70: 221–227.
Stachowiak MK, Kucinski A, Curl R, Syposs C, Yang Y, Narla S et al. Schizophrenia: a neurodevelopmental disorder – integrative genomic hypothesis and therapeutic implications from a transgenic mouse model. Schizophr Res 2013; 143: 367–376.
Mahieu-Caputo D, Sonigo P, Amiel J, Simon I, Aubry MC, Lemerrer M et al. Prenatal diagnosis of sporadic Apert syndrome: a sequential diagnostic approach combining three-dimensional computed tomography and molecular biology. Fetal Diagn Ther. 2001; 16: 10–12.
Padmanabhan V, Hegde AM, Rai K . Crouzon's syndrome: a review of literature and case report. Contemp Clin Dent 2011; 2: 211–214.
Wentz E, Vujic M, Karrstedt EL, Erlandsson A, Gillberg C . A case report of two male siblings with autism and duplication of Xq13-q21, a region including three genes predisposing for autism. European Child Adolesc Psychiatry 2014; 23: 329–336.
Kaga Y, Shoemaker WJ, Furusho M, Bryant M, Rosenbluth J, Pfeiffer SE et al. Mice with conditional inactivation of fibroblast growth factor receptor-2 signaling in oligodendrocytes have normal myelin but display dramatic hyperactivity when combined with Cnp1 inactivation. J Neurosci. 2006; 26: 12339–12350.
O'Donovan MC, Norton N, Williams H, Peirce T, Moskvina V, Nikolov I et al. Analysis of 10 independent samples provides evidence for association between schizophrenia and a SNP flanking fibroblast growth factor receptor 2. Mol Psychiatry 2009; 14: 30–36.
Stevens HE, Su T, Yanagawa Y, Vaccarino FM . Prenatal stress delays inhibitory neuron progenitor migration in the developing neocortex. Psychoneuroendocrinology 2013; 38: 509–521.
Theisen A, Rosenfeld JA, Shane K, McBride KL, Atkin JF, Gaba C et al. Refinement of the region for split hand/foot malformation 5 on 2q31.1. Mol Syndromol 2010; 1: 262–271.
Liu X, Novosedlik N, Wang A, Hudson ML, Cohen IL, Chudley AE et al. The DLX1and DLX2 genes and susceptibility to autism spectrum disorders. Eur J Hum Genet 2009; 17: 228–235.
Uchida T, Furukawa T, Iwata S, Yanagawa Y, Fukuda A . Selective loss of parvalbumin-positive GABAergic interneurons in the cerebral cortex of maternally stressed Gad1-heterozygous mouse offspring. Transl Psychiatry 2014; 4: e371.
Lazarus MS, Krishnan K, Huang ZJ . GAD67 Deficiency in parvalbumin interneurons produces deficits in inhibitory transmission and network disinhibition in mouse prefrontal cortex. Cereb Cortex 2013 (e-pub ahead of print).
Bacchelli E, Blasi F, Biondolillo M, Lamb JA, Bonora E, Barnby G et al. Screening of nine candidate genes for autism on chromosome 2q reveals rare nonsynonymous variants in the cAMP-GEFII gene. Mol Psychiatry 2003; 8: 916–924.
Rabionet R, Jaworski JM, Ashley-Koch AE, Martin ER, Sutcliffe JS, Haines JL et al. Analysis of the autism chromosome 2 linkage region: GAD1 and other candidate genes. Neurosci Lett. 2004; 372: 209–214.
Buttenschon HN, Lauritsen MB, El Daoud A, Hollegaard M, Jorgensen M, Tvedegaard K et al. A population-based association study of glutamate decarboxylase 1 as a candidate gene for autism. J Neural Transm. 2009; 116: 381–388.
Chang SC, Pauls DL, Lange C, Sasanfar R, Santangelo SL . Common genetic variation in the GAD1 gene and the entire family of DLX homeobox genes and autism spectrum disorders. Am J Med Genet B Neuropsychiatr Genet 2011; 156: 233–239.
Akbarian S, Huang HS . Molecular and cellular mechanisms of altered GAD1/GAD67 expression in schizophrenia and related disorders. Brain Res Rev 2006; 52: 293–304.
Ayalew M, Le-Niculescu H, Levey DF, Jain N, Changala B, Patel SD et al. Convergent functional genomics of schizophrenia: from comprehensive understanding to genetic risk prediction. Mol Psychiatry 2012; 17: 887–905.
Straub RE, Lipska BK, Egan MF, Goldberg TE, Callicott JH, Mayhew MB et al. Allelic variation in GAD1 (GAD67) is associated with schizophrenia and influences cortical function and gene expression. Mol Psychiatry 2007; 12: 854–869.
Cherlyn SY, Woon PS, Liu JJ, Ong WY, Tsai GC, Sim K . Genetic association studies of glutamate, GABA and related genes in schizophrenia and bipolar disorder: a decade of advance. Neurosci Biobehav Rev 2010; 34: 958–977.
Cooper MA, Koleske AJ . Ablation of ErbB4 from excitatory neurons leads to reduced dendritic spine density in mouse prefrontal cortex. J Comp Neurol 2014; 522: 3351–3362.
Ting AK, Chen Y, Wen L, Yin DM, Shen C, Tao Y et al. Neuregulin 1 promotes excitatory synapse development and function in GABAergic interneurons. J Neurosci. 2011; 31: 15–25.
Hou XJ, Ni KM, Yang JM, Li XM . Neuregulin 1/ErbB4 enhances synchronized oscillations of prefrontal cortex neurons via inhibitory synapses. Neuroscience 2014; 261: 107–117.
Yang JM, Zhang J, Chen XJ, Geng HY, Ye M, Spitzer NC et al. Development of GABA circuitry of fast-spiking basket interneurons in the medial prefrontal cortex of erbb4-mutant mice. J Neurosci. 2013; 33: 19724–19733.
Kasnauskiene J, Ciuladaite Z, Preiksaitiene E, Utkus A, Peciulyte A, Kucinskas V . A new single gene deletion on 2q34: ERBB4 is associated with intellectual disability. Am J Med Genet 2013; 161A: 1487–1490.
Joshi D, Fullerton JM, Weickert CS . Elevated ErbB4 mRNA is related to interneuron deficit in prefrontal cortex in schizophrenia. J Psychiatr Res. 2014; 53: 125–132.
Del Pino I, Garcia-Frigola C, Dehorter N, Brotons-Mas JR, Alvarez-Salvado E, Martinez de Lagran M et al. Erbb4 deletion from fast-spiking interneurons causes schizophrenia-like phenotypes. Neuron 2013; 79: 1152–1168.
Buonanno A . The neuregulin signaling pathway and schizophrenia: from genes to synapses and neural circuits. Brain Res Bull 2010; 83: 122–131.
Lang UE, Puls I, Muller DJ, Strutz-Seebohm N, Gallinat J . Molecular mechanisms of schizophrenia. Cell Physiol Biochem. 2007; 20: 687–702.
Yonan AL, Palmer AA, Smith KC, Feldman I, Lee HK, Yonan JM et al. Bioinformatic analysis of autism positional candidate genes using biological databases and computational gene network prediction. Genes Brain Behav 2003; 2: 303–320.
Yonan AL, Alarcon M, Cheng R, Magnusson PK, Spence SJ, Palmer AA et al. A genomewide screen of 345 families for autism-susceptibility loci. Am J Hum Genet 2003; 73: 886–897.
Neves-Pereira M, Muller B, Massie D, Williams JH, O'Brien PC, Hughes A et al. Deregulation of EIF4E: a novel mechanism for autism. J Med Genet 2009; 46: 759–765.
Gkogkas CG, Sonenberg N . Translational control and autism-like behaviors. Cell Logist 2013; 3: e24551.
Krueger DD, Osterweil EK, Chen SP, Tye LD, Bear MF . Cognitive dysfunction and prefrontal synaptic abnormalities in a mouse model of fragile X syndrome. Proc Natl Acad Sci USA. 2011; 108: 2587–2592.
Bhakar AL, Dolen G, Bear MF . The pathophysiology of fragile X (and what it teaches us about synapses). Annu Rev Neurosci 2012; 35: 417–443.
Irwin SA, Galvez R, Greenough WT . Dendritic spine structural anomalies in fragile-X mental retardation syndrome. Cereb Cortex. 2000; 10: 1038–1044.
De Rubeis S, Fernandez E, Buzzi A, Di Marino D, Bagni C . Molecular and cellular aspects of mental retardation in the Fragile X syndrome: from gene mutation/s to spine dysmorphogenesis. Adv Exp Med Biol 2012; 970: 517–551.
Maurin T, Zongaro S, Bardoni B . Fragile X Syndrome: from molecular pathology to therapy. Neurosci Biobehav Rev 2014 (in press).
Rotschafer SE, Razak KA . Auditory processing in fragile x syndrome. Front Cell Neurosci 2014; 8: 19.
Oddi D, Crusio WE, D'Amato FR, Pietropaolo S . Monogenic mouse models of social dysfunction: implications for autism. Behav Brain Res 2013; 251: 75–84.
Tranfaglia MR . The psychiatric presentation of fragile x: evolution of the diagnosis and treatment of the psychiatric comorbidities of fragile X syndrome. Dev Neurosci 2011; 33: 337–348.
Kramvis I, Mansvelder HD, Loos M, Meredith R . Hyperactivity, perseveration and increased responding during attentional rule acquisition in the Fragile X mouse model. Front Behav Neurosci 2013; 7: 172.
Kim L, He L, Maaswinkel H, Zhu L, Sirotkin H, Weng W . Anxiety, hyperactivity and stereotypy in a zebrafish model of fragile X syndrome and autism spectrum disorder. Prog Neuropsychopharmacol Biol Psychiatry 2014; 55: 40–49.
Kraan CM, Hocking DR, Georgiou-Karistianis N, Metcalfe SA, Archibald AD, Fielding J et al. Impaired response inhibition is associated with self- reported symptoms of depression, anxiety, and ADHD in female FMR1 premutation carriers. Am J Med Genet B Neuropsychiatr Genet 2014; 165: 41–51.
Hunter JE, Epstein MP, Tinker SW, Abramowitz A, Sherman SL . The FMR1 premutation and attention-deficit hyperactivity disorder (ADHD): evidence for a complex inheritance. Behav Genet 2012; 42: 415–422.
Kovacs T, Kelemen O, Keri S . Decreased fragile X mental retardation protein (FMRP) is associated with lower IQ and earlier illness onset in patients with schizophrenia. Psychiatry Res. 2013; 210: 690–693.
Fatemi SH, Folsom TD . The role of fragile X mental retardation protein in major mental disorders. Neuropharmacology 2011; 60: 1221–1226.
Fromer M, Pocklington AJ, Kavanagh DH, Williams HJ, Dwyer S, Gormley P et al. De novo mutations in schizophrenia implicate synaptic networks. Nature 2014; 506: 179–184.
Yadav R, Gupta SC, Hillman BG, Bhatt JM, Stairs DJ, Dravid SM . Deletion of glutamate delta-1 receptor in mouse leads to aberrant emotional and social behaviors. PloS One 2012; 7: e32969.
Griswold AJ, Ma D, Cukier HN, Nations LD, Schmidt MA, Chung RH et al. Evaluation of copy number variations reveals novel candidate genes in autism spectrum disorder-associated pathways. Hum Mol Genet 2012; 21: 3513–3523.
Guo SZ, Huang K, Shi YY, Tang W, Zhou J, Feng GY et al. A case-control association study between the GRID1 gene and schizophrenia in the Chinese Northern Han population. Schizophr Res 2007; 93: 385–390.
Treutlein J, Muhleisen TW, Frank J, Mattheisen M, Herms S, Ludwig KU et al. Dissection of phenotype reveals possible association between schizophrenia and Glutamate Receptor Delta 1 (GRID1) gene promoter. Schizophr Res 2009; 111: 123–130.
Kolk SM, Gunput RA, Tran TS, van den Heuvel DM, Prasad AA, Hellemons AJ et al. Semaphorin 3F is a bifunctional guidance cue for dopaminergic axons and controls their fasciculation, channeling, rostral growth, and intracortical targeting. J Neurosci. 2009; 29: 12542–12557.
Brooks-Kayal A . Molecular mechanisms of cognitive and behavioral comorbidities of epilepsy in children. Epilepsia 2011; 52: 13–20.
Wu S, Yue W, Jia M, Ruan Y, Lu T, Gong X et al. Association of the neuropilin-2 (NRP2) gene polymorphisms with autism in Chinese Han population. Am J Med Genet B Neuropsychiatr Genet 2007; 144B: 492–495.
Iafrati J, Orejarena MJ, Lassalle O, Bouamrane L, Gonzalez-Campo C, Chavis P . Reelin, an extracellular matrix protein linked to early onset psychiatric diseases, drives postnatal development of the prefrontal cortex via GluN2B-NMDARs and the mTOR pathway. Mol Psychiatry 2014; 19: 417–426.
Zaki M, Shehab M, El-Aleem AA, Abdel-Salam G, Koeller HB, Ilkin Y et al. Identification of a novel recessive RELN mutation using a homozygous balanced reciprocal translocation. Am J Med Genet 2007; 143A: 939–944.
Folsom TD, Fatemi SH . The involvement of Reelin in neurodevelopmental disorders. Neuropharmacology 2013; 68: 122–135.
Bartlett CW, Gharani N, Millonig JH, Brzustowicz LM . Three autism candidate genes: a synthesis of human genetic analysis with other disciplines. Int J Dev Neurosci. 2005; 23: 221–234.
Fatemi SH . The role of Reelin in pathology of autism. Mol Psychiatry 2002; 7: 919–920.
Fatemi SH . Reelin glycoprotein in autism and schizophrenia. Int Rev Neurobiol. 2005; 71: 179–187.
Lakatosova S, Ostatnikova D . Reelin and its complex involvement in brain development and function. Int J Biochem Cell Biol. 2012; 44: 1501–1504.
Frotscher M . Role for Reelin in stabilizing cortical architecture. Trends Neurosci 2010; 33: 407–414.
Zhang W, Peterson M, Beyer B, Frankel WN, Zhang ZW . Loss of MeCP2 from forebrain excitatory neurons leads to cortical hyperexcitation and seizures. J Neurosci. 2014; 34: 2754–2763.
Francke U . Mechanisms of disease: neurogenetics of MeCP2 deficiency. Nat Clin Pract Neurol. 2006; 2: 212–221.
Samaco RC, Neul JL . Complexities of Rett syndrome and MeCP2. J Neurosci. 2011; 31: 7951–7959.
Lanz TA, Guilmette E, Gosink MM, Fischer JE, Fitzgerald LW, Stephenson DT et al. Transcriptomic analysis of genetically defined autism candidate genes reveals common mechanisms of action. Mol Autism 2013; 4: 45.
Suter B, Treadwell-Deering D, Zoghbi HY, Glaze DG, Neul JL . Brief report: MECP2 mutations in people without Rett syndrome. J Autism Dev Disord 2014; 44: 703–711.
Neul JL . The relationship of Rett syndrome and MECP2 disorders to autism. Dialogues Clin Neurosci 2012; 14: 253–262.
McCarthy SE, Gillis J, Kramer M, Lihm J, Yoon S, Berstein Y et al. De novo mutations in schizophrenia implicate chromatin remodeling and support a genetic overlap with autism and intellectual disability. Mol Psychiatry 2014; 19: 652–658.
Piton A, Gauthier J, Hamdan FF, Lafreniere RG, Yang Y, Henrion E et al. Systematic resequencing of X-chromosome synaptic genes in autism spectrum disorder and schizophrenia. Mol Psychiatry 2011; 16: 867–880.
Xu B, Goldman JS, Rymar VV, Forget C, Lo PS, Bull SJ et al. Critical roles for the netrin receptor deleted in colorectal cancer in dopaminergic neuronal precursor migration, axon guidance, and axon arborization. Neuroscience 2010; 169: 932–949.
Manitt C, Eng C, Pokinko M, Ryan RT, Torres-Berrio A, Lopez JP et al. Dcc orchestrates the development of the prefrontal cortex during adolescence and is altered in psychiatric patients. Transl Psychiatry 2013; 3: e338.
Grant A, Fathalli F, Rouleau G, Joober R, Flores C . Association between schizophrenia and genetic variation in DCC: a case-control study. Schizophr Res. 2012; 137: 26–31.
El-Hassar L, Simen AA, Duque A, Patel KD, Kaczmarek LK, Arnsten AF et al. Disrupted in schizophrenia 1 modulates medial prefrontal cortex pyramidal neuron activity through cAMP regulation of transient receptor potential C and small-conductance K channels. Biol Psychiatry 2014; 76: 476–485.
Kilpinen H, Ylisaukko-Oja T, Hennah W, Palo OM, Varilo T, Vanhala R et al. Association of DISC1 with autism and Asperger syndrome. Mol Psychiatry 2008; 13: 187–196.
Zheng F, Wang L, Jia M, Yue W, Ruan Y, Lu T et al. Evidence for association between disrupted-in-Schizophrenia 1 (DISC1) gene polymorphisms and autism in Chinese Han population: a family-based association study. Behav Brain Funct. 2011; 7: 14.
Kenny EM, Cormican P, Furlong S, Heron E, Kenny G, Fahey C et al. Excess of rare novel loss-of-function variants in synaptic genes in schizophrenia and autism spectrum disorders. Mol Psychiatry 2013; 19: 872–879.
Kalkman HO . A review of the evidence for the canonical Wnt pathway in autism spectrum disorders. Mol Autism 2012; 3: 10.
Jacobsen KK, Halmoy A, Sanchez-Mora C, Ramos-Quiroga JA, Cormand B, Haavik J et al. DISC1 in adult ADHD patients: an association study in two European samples. Am J Med Genet B Neuropsychiatr Genet 2013; 162B: 227–234.
Narayan S, Nakajima K, Sawa A . DISC1: a key lead in studying cortical development and associated brain disorders. Neuroscientist 2013; 19: 451–464.
Hikida T, Gamo NJ, Sawa A . DISC1 as a therapeutic target for mental illnesses. Expert Opin Ther Targets. 2012; 16: 1151–1160.
Duff BJ, Macritchie KA, Moorhead TW, Lawrie SM, Blackwood DH . Human brain imaging studies of DISC1 in schizophrenia, bipolar disorder and depression: a systematic review. Schizophr Res 2013; 147: 1–13.
Drerup JM, Hayashi K, Cui H, Mettlach GL, Long MA, Marvin M et al. Attention-deficit/hyperactivity phenotype in mice lacking the cyclin- dependent kinase 5 cofactor p35. Biol Psychiatry. 2010; 68: 1163–1171.
Ramos-Miguel A, Meana JJ, Garcia-Sevilla JA . Cyclin-dependent kinase-5 and p35/p25 activators in schizophrenia and major depression prefrontal cortex: basal contents and effects of psychotropic medications. Int J Neuropsychopharmacol. 2013; 16: 683–689.
Engmann O, Hortobagyi T, Pidsley R, Troakes C, Bernstein HG, Kreutz MR et al. Schizophrenia is associated with dysregulation of a Cdk5 activator that regulates synaptic protein expression and cognition. Brain 2011; 134: 2408–2421.
Whitwell JL, Josephs KA, Avula R, Tosakulwong N, Weigand SD, Senjem ML et al. Altered functional connectivity in asymptomatic MAPT subjects: a comparison to bvFTD. Neurology 2011; 77: 866–874.
Gregor A, Krumbiegel M, Kraus C, Reis A, Zweier C . De novo triplication of the MAPT gene from the recurrent 17q21.31 microdeletion region in a patient with moderate intellectual disability and various minor anomalies. Am J Med Genet A 2012; 158A: 1765–1770.
Rovelet-Lecrux A, Campion D . Copy number variations involving the microtubule-associated protein tau in human diseases. Biochem Soc Trans. 2012; 40: 672–676.
Sapir T, Frotscher M, Levy T, Mandelkow EM, Reiner O . Tau's role in the developing brain: implications for intellectual disability. Hum Mol Genet. 2012; 21: 1681–1692.
Kitsiou-Tzeli S, Frysira H, Giannikou K, Syrmou A, Kosma K, Kakourou G et al. Microdeletion and microduplication 17q21.31 plus an additional CNV, in patients with intellectual disability, identified by array-CGH. Gene 2012; 492: 319–324.
Runker AE, O'Tuathaigh C, Dunleavy M, Morris DW, Little GE, Corvin AP et al. Mutation of Semaphorin-6A disrupts limbic and cortical connectivity and models neurodevelopmental psychopathology. PloS One 2011; 6: e26488.
Bosia M, Anselmetti S, Pirovano A, Ermoli E, Marino E, Bramanti P et al. HTTLPR functional polymorphism in schizophrenia: executive functions versus sustained attention dissociation. Prog Neuropsychopharmacol Biol Psychiatry 2010; 34: 81–85.
Lin C, Tang W, Hu J, Gao L, Huang K, Xu Y et al. Haplotype analysis confirms association of the serotonin transporter (5-HTT) gene with schizophrenia in the Han Chinese population. Neurosci Lett. 2009; 453: 210–213.
Acknowledgements
This work was supported by grants from the Donders Centre for Neuroscience, Radboud University Nijmegen (DS, SMK). The authors thank Prof B. Franke, Dr W. Scheenen, Prof H. van Bokhoven and Prof A. Kriegstein for critically reviewing the manuscript and the anonymous reviewers for their comments. Also, we apologize for those primary works not referenced here due to space limitations.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
This work is licensed under a Creative Commons Attribution 4.0 International License. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in the credit line; if the material is not included under the Creative Commons license, users will need to obtain permission from the license holder to reproduce the material. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/
About this article
Cite this article
Schubert, D., Martens, G. & Kolk, S. Molecular underpinnings of prefrontal cortex development in rodents provide insights into the etiology of neurodevelopmental disorders. Mol Psychiatry 20, 795–809 (2015). https://doi.org/10.1038/mp.2014.147
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/mp.2014.147
This article is cited by
-
Brain-derived neurotrophic factor from microglia regulates neuronal development in the medial prefrontal cortex and its associated social behavior
Molecular Psychiatry (2024)
-
Loss of spines in the prelimbic cortex is detrimental to working memory in mice with early-life adversity
Molecular Psychiatry (2023)
-
Neurogenesis and neuronal differentiation in the postnatal frontal cortex in Down syndrome
Acta Neuropathologica Communications (2022)
-
Ontogeny of the Corticolimbic System and the Risk of Anxiety Disorders in Adolescence
Neuroscience and Behavioral Physiology (2022)
-
Behavioral, neuroanatomical, and molecular correlates of resilience and susceptibility to maternal immune activation
Molecular Psychiatry (2021)