Abstract
The current College of American Pathologists cancer template for reporting biopsies of bone tumors recommends including information that is of unproven prognostic significance for osteosarcoma, such as the presence of spontaneous tumor necrosis and mitotic rate. Conversely, the degree of cytologic anaplasia (degree of differentiation) is not reported in this template. This retrospective cohort study of 125 patients with high-grade osteosarcoma was performed to evaluate the prognostic impact of these factors in diagnostic biopsy specimens in predicting the clinical outcome and response to neoadjuvant chemotherapy. Multivariate Cox regression was performed to adjust survival analyses for well-established prognostic factors. Multivariate logistic regression was used to determine odds ratios for good chemotherapy response (≥90% tumor necrosis). Osteosarcomas with severe anaplasia were independently associated with increased overall and disease-free survival, but mitotic rate and spontaneous necrosis had no prognostic impact after controlling for other confounding factors. Mitotic rate showed a trend towards increased odds of a good histologic response, but this effect was diminished after controlling for other predictive factors. Neither spontaneous necrosis nor the degree of cytologic anaplasia observed in biopsy specimens was predictive of a good response to chemotherapy. Mitotic rate and spontaneous tumor necrosis observed in pretreatment biopsy specimens of high-grade osteosarcoma are not strong independent prognostic factors for clinical outcome or predictors of response to neoadjuvant chemotherapy. Therefore, reporting these parameters for osteosarcoma, as recommended in the College of American Pathologists Bone Biopsy template, does not appear to have clinical utility. In contrast, histologic grading schemes for osteosarcoma based on the degree of cytologic anaplasia may have independent prognostic value and should continue to be evaluated.
Similar content being viewed by others
Main
The current College of American Pathologists cancer reporting template for biopsies of bone tumors requires including information that is of unclear diagnostic or prognostic significance, such as the presence of spontaneous tumor necrosis (endogenous tumor necrosis seen in cytotoxic therapy-naive tumor samples) and mitotic rate.1 There has been limited evaluation of the clinical significance of these parameters in biopsy specimens obtained before administration of cytotoxic chemotherapy. Furthermore, the prognostic importance of cytologic anaplasia beyond classification of osteosarcoma as low grade (parosteal and low-grade central types), intermediate grade (periosteal), or high grade (conventional and other special types) is also controversial.
Even if these factors provide limited prognostic information regarding the clinical outcome, they might be useful to predict the response to adjuvant chemotherapy, which is arguably the most important prognostic factor for osteosarcoma besides the presence of metastatic disease.2, 3 Therefore, this study was performed to determine the prognostic impact of mitotic rate, spontaneous tumor necrosis, and degree of cytologic anaplasia in diagnostic biopsy specimens of high-grade osteosarcoma. In addition, the relationships of these pathologic characteristics to chemotherapy response were also examined.
Materials and methods
Patients
The Surgical Pathology files at Vanderbilt University Medical Center were searched for biopsy specimens from patients with high-grade osteosarcoma whose original diagnosis was made at Vanderbilt and who subsequently received neoadjuvant chemotherapy and underwent surgical resection between 1985 and 2010. Osteosarcomas of low (parosteal and low-grade central osteosarcoma) or intermediate grade (periosteal osteosarcoma) were excluded. A total of 143 cases were identified; unusual histologic types of osteosarcoma (2 small cell osteosarcomas, 2 high-grade surface tumors, and 1 dedifferentiated parosteal osteosarcoma) were excluded. Slides from eight diagnostic biopsy specimens could not be located and histologic response to neoadjuvant chemotherapy-induced tumor necrosis could not be determined in 5 cases, leaving 125 cases for further study. Of these, 123 were incisional biopsies and 2 were core biopsies.
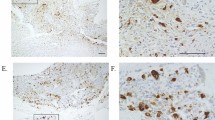
Pathology reports from both biopsy and resection specimens were reviewed and the anatomic location and size of the tumor, histologic diagnosis, and subtype according to current WHO criteria,4, 5 Musculoskeletal Tumor Society stage,6 and status of surgical resection margins were recorded. All H&E-stained slides from the biopsy specimens were reviewed by to record the overall percent of spontaneous necrosis present and mitotic rate (mitotic figures per 10 high-power fields; × 400 or 0.1734 mm2). Degree of cytologic atypia/anaplasia was scored as mild, moderate, or severe based on nucleomegaly, nuclear hyperchromasia, and nuclear pleomorphism according to the Mirra’s criteria.7 In brief, tumors with mild cytologic atypia/anaplasia were relatively monomorphic, with minimal anisonucleosis and subtle nuclear irregularity requiring examination at high power to confirm the cytologic diagnosis of malignancy. In contrast, those with severe cytologic atypia/anaplasia contained large and bizarre tumor giant cells easily identified at scanning magnification (Figure 1). Neoadjuvant chemotherapy-induced tumor necrosis was estimated through review of all slides from subsequent surgical resection specimens using standard technique.8 Clinical data were abstracted from electronic medical records.
Median follow-up for censored patients was 109 months (range, 1 day–339 months). There were 54 deaths due to progressive disease, with a median disease-specific survival of 23 months after surgical resection (range, 3–127 months). An additional 11 patients developed metastasis, but were alive with disease at last clinical follow-up (median disease-free survival, 17 months; range, 1–190 months). The study protocol was approved by the Vanderbilt University Institutional Review Board (#120193; 02/07/2012); a waiver of informed consent was obtained.
Statistical Analysis
Clinicopathologic variables were compared among study groups using standard bivariate statistical tests; categorical variables were compared using Fisher’s exact test and continuous variables were compared using Student’s t-test with unequal variances. Survival analyses were performed using Cox proportional hazard regression. Variables other than those of specific interest were included in multivariate regression models if P≤0.1 from univariate regressions. Logistic regression was used to assess odds ratios for predictive variables; those with statistically significant odds ratios in univariate regression (P≤0.05) were included in a multivariate logistic regression model. For logistic regression, six outlier cases with mitotic rates >50 per 10 high-power fields were recoded as having 50 mitotic figures per 10 high-power fields. Variables considered for inclusion in Cox and logistic regression models are listed in Table 1. All tests were two-sided with α=0.05 and all statistical analyses were performed using Stata v13.1 (StataCorp, College Station, TX, USA).
Results
Clinicopathologic characteristics by response to neoadjuvant chemotherapy (<90% or ≥90% chemotherapy-induced tumor necrosis) are presented in Table 1. As expected, patients with good responses tended to be younger and had tumors involving the appendicular skeleton. Advanced tumor stage and non-osteoblastic tumors also showed trends towards decreased histologic response to chemotherapy. Poorly responsive tumors were incompletely resected significantly more often than those with a good histologic response. In addition, tumors with higher mitotic rates were also associated with good histologic response. Cytologic anaplasia and spontaneous necrosis were not associated with good histologic response in standard bivariate statistical tests.
The percentage of chemotherapy-induced tumor necrosis (evaluated as a continuous variable) was not significantly different among tumors with varying degrees of cytologic anaplasia (ANOVA, F=0.51; P=0.60). Correlation analysis further showed a positive relationship between mitotic rate and extent of chemotherapy-induced tumor necrosis (Kendall’s τA=0.175; P=0.003). Similar analyses failed to demonstrate correlations between extents of spontaneous and chemotherapy-induced tumor necrosis (Kendall’s τA=0.049; P=0.38) or spontaneous tumor necrosis and mitotic rate (Kendall’s τA=0.015; P=0.79).
Survival Analysis
In univariate Cox regression models, advanced Musculoskeletal Tumor Society stage, poor histologic response to chemotherapy, increasing tumor size, non-appendicular anatomic location, positive surgical resection margins, and the telangiectatic subtype of osteosarcoma were associated with increased hazard ratios for both overall and disease-free survival (Table 2). Therefore, these variables were included in subsequent multivariate proportional hazard regression models (see below). Tumor size was excluded from the multivariate model because of collinearity with Musculoskeletal Tumor Society stage, as these two variables were strongly associated with one another (ANOVA, F=14.39; P<0.0005).
Severely anaplastic tumors showed decreased hazard ratios in univariate analyses for both overall and disease-free survival compared with those with mild anaplasia. Increasing mitotic rate showed a trend towards longer overall survival, but not disease-free survival. Extent of spontaneous necrosis did not show statistically significant associations with clinical outcomes in univariate analysis (Table 2).
Severe cytologic anaplasia was independently associated with decreased hazard ratios for both overall survival and disease-free survival after controlling for confounding factors in multivariate Cox regression (Table 3; Figure 2). In contrast, neither spontaneous tumor necrosis nor mitotic rate showed significant associations with the clinical outcome in multivariate regression models (Table 3; Supplementary Figures 1 and 2).
Overall survival curves of patients with high-grade osteosarcoma who showed either poor histologic response (<90% tumor necrosis) or good histologic response (≥90% tumor necrosis) to neoadjuvant chemotherapy grouped by the degree of cytologic anaplasia, as determined in diagnostic biopsy specimens. Survival curves are adjusted for Musculoskeletal Tumor Society stage (non-metastatic), anatomic site (appendicular), and histologic subtype (non-telangiectatic). Results from Wald tests that the hazards within each subgroup are identical after adjusting for confounding covariates are also provided.
Association With Chemotherapy Response
As chemotherapy response is such a powerful prognostic factor for outcomes in osteosarcoma, we next determined whether mitotic rate, spontaneous necrosis, or degree of cytologic anaplasia observed in biopsy specimens were predictive of a good histologic response to neoadjuvant chemotherapy (≥90% tumor necrosis) in subsequent resection specimens. Increasing age, anatomic location in the pelvis, scapula, or craniofacial bones, the presence of metastatic disease at diagnosis, and increasing mitotic rate were all significantly associated with decreased odds of good chemotherapy response in univariate logistic regression (Table 4). In addition, the chondroblastic subtype of osteosarcoma also showed marginally significant increased odds for a good response. Neither extent of spontaneous necrosis nor cytologic anaplasia showed significant odds ratios in univariate analyses.
After controlling for patient age, anatomic site, Musculoskeletal Tumor Society stage, and histologic subtype,9, 10, 11 mitotic rate, extent of spontaneous necrosis, or degree of cytologic anaplasia were not statistically significant predictors of good histologic response to neoadjuvant chemotherapy in multivariate logistic regression (Table 5). The effects of each of these factors on the probability of a good response in patients with otherwise favorable prognostic factors (localized, appendicular, and osteoblastic osteosarcoma) while holding age constant at its median value are demonstrated graphically in Figures 3,4,5. For comparison, the effects of statistically significant predictive factors for histologic response to neoadjuvant chemotherapy (patient age, Musculoskeletal Tumor Society stage, histologic subtype, and anatomic site) are provided in Supplementary Figures 3.
Histologic response to neoadjuvant chemotherapy (% tumor necrosis) by extent of spontaneous tumor necrosis observed in diagnostic biopsy specimens of high-grade osteosarcoma. Predicted probabilities (black line) and 95% confidence intervals (gray band) of achieving a good histologic response from a multivariate logistic regression model adjusted for median patient age (17.4 years), Musculoskeletal Tumor Society stage (non-metastatic), anatomic site (appendicular), and histologic subtype (osteoblastic osteosarcoma) are plotted over extent of spontaneous tumor necrosis. Data from individual patients are also shown (points).
Histologic response to neoadjuvant chemotherapy (% tumor necrosis) by the degree of cytologic anaplasia observed in diagnostic biopsy specimens of high-grade osteosarcoma. Predicted probabilities (black diamonds) and 95% confidence intervals of achieving a good histologic response from a multivariate logistic regression model adjusted for median patient age (17.4 years), Musculoskeletal Tumor Society stage (non-metastatic), anatomic site (appendicular), and histologic subtype (osteoblastic osteosarcoma) are plotted by the degree of cytologic anaplasia. Data from individual patients are also shown (points).
Histologic response to neoadjuvant chemotherapy (% tumor necrosis) by mitotic rate (mitotic figures per 10 high-power fields) observed in diagnostic biopsy specimens of high-grade osteosarcoma. Predicted probabilities (black line) and 95% confidence intervals (gray band) of achieving a good histologic response from a multivariate logistic regression model adjusted for median patient age (17.4 years), Musculoskeletal Tumor Society stage (non-metastatic), anatomic site (appendicular), and histologic subtype (osteoblastic osteosarcoma) are plotted over mitotic rate. Data from individual patients are also shown (points).
Discussion
The results of this study suggest that the degree of cytologic anaplasia in high-grade osteosarcoma is an independent prognostic factor for overall and disease-free survival, but mitotic rate and spontaneous necrosis are not. Despite an association between mitotic rate and good histologic response to neoadjuvant chemotherapy in univariate analyses, the strength of this association diminished after controlling for other important confounding factors, such as Musculoskeletal Tumor Society stage, anatomic site, histologic subtype of osteosarcoma, and the age of the patient. Similarly, cytologic anaplasia and extent of spontaneous tumor necrosis observed in pretreatment biopsy specimens were not statistically significant predictive factors for histologic response to neoadjuvant chemotherapy in this cohort.
There has been limited evaluation of the clinical significance of spontaneous tumor necrosis observed before administration of cytotoxic chemotherapy. Whereas some evidence shows an association with decreased survival in patients—at least in those not treated with chemotherapy,12, 13 a more recent study suggests that measuring necrosis in pretreatment biopsies has no prognostic significance.14 Similarly, data regarding proliferation rate (as measured by Ki67 labeling index) are conflicting, with some reports showing increased overall or metastasis-free survival with lower labeling indices,15, 16, 17 and others showing no association.18, 19, 20
The importance of cytologic anaplasia in prognosis of osteosarcoma is also controversial. Currently, osteosarcomas are broadly classified by histologic type as low, intermediate, and high grade, with all conventional osteosarcomas considered high grade by definition. This is not particularly useful for risk stratification, and other grading schemes should continue to be evaluated. Price21 originally reported that osteosarcomas with high-grade cytologic atypia were associated with poor prognosis. But this was before treatment with neoadjuvant chemotherapy became standard of care. Other authors have since failed to observe this association.22, 23, 24 Here we found a significant trend for increased survival in patients with severely anaplastic osteosarcomas treated with neoadjuvant chemotherapy compared with tumors with more monomorphic cytology. Although this result might seem counterintuitive, a greater proportion of severely anaplastic osteosarcomas showed a good histologic response to chemotherapy than did tumors with mild or moderate anaplasia (79% vs 59–61%; Table 1). This difference failed to reach statistical significance by logistic regression, possibly because of the limited number of severely anaplastic tumors in this cohort (N=19).
One possible explanation for the results of this study is sampling error, given the intratumoral heterogeneity for these factors in osteosarcoma. A core or incisional biopsy is typically a very restricted sample of the entire tumor, and the proliferative fraction, extent of tumor necrosis, and even the degree of cytologic atypia observed in tissue obtained at biopsy is unlikely to be representative of the entire tumor.19, 25 Indeed, this sampling issue is perhaps the most important reason why these factors should not be conveyed in pathology reports for osteosarcoma biopsy specimens. Another explanation is insufficient study power given the limited sample size available for analysis. Nonetheless, the sample size is sufficient to conclude that mitotic rate and spontaneous necrosis are not strong independent predictive factors of patient outcome, particularly in comparison with other well-established prognostic factors.
Although this study did not consider the clinical importance of reporting these variables in osteosarcoma resection specimens, as recommended in the College of American Pathologists Bone Tumor Resection template,1 these variables have even less clinical relevance in this setting. Mitotic activity observed in osteosarcomas resected after neoadjuvant chemotherapy would most likely represent a surrogate marker for the amount of residual viable osteosarcoma, and be inversely related to histologic response to chemotherapy. Obviously, it is not possible to evaluate the presence of spontaneous tumor necrosis after the administration of neoadjuvant cytotoxic therapy. Similarly, cytologic anaplasia cannot be evaluated in this setting, as tumor cytomorphology is often profoundly altered after neoadjuvant chemotherapy.
One argument for continuing to report these variables in the College of American Pathologists Bone Tumor template is that these factors are used to histologically grade osseous sarcomas that typically arise in soft tissue (by the Fédération Nationale des Centres de Lutte Contre le Cancer system). However, these cases account for <15% of skeletal sarcomas based on the SEER data.26 In contrast, osteosarcoma and chondrosarcoma make up almost 75% of tumors reportable in the College of American Pathologists Bone Tumor template (Ewing’s sarcoma/primitive neuroectodermal tumor is reported using a separate template customized for this specific tumor type), which prompts the question of whether pathologists should continue using a generic template that includes irrelevant diagnostic and prognostic information for most skeletal sarcomas. One suggestion would be to design separate reporting templates for the most common primary tumors of bone and use the generic template for other less common skeletal sarcomas.
The findings presented here raise important questions concerning the utility of reporting the extent of spontaneous tumor necrosis and mitotic rate for biopsies of high-grade osteosarcoma, as required in the College of American Pathologists Bone Biopsy and Resection templates,1 particularly in regard to the potential for sampling error in limited biopsy specimens. There is a marked paucity of evidence for the clinical relevance of some of the required data elements in the current College of American Pathologists protocol for reporting biopsies and resections of bone tumors. Until more data are collected, there appears to be little value in recording these parameters. Instead, further study of cytologic grading schemes for osteosarcoma may be warranted to determine whether the degree of cytologic anaplasia observed before exposure to neoadjuvant chemotherapy is associated with the clinical outcome.
References
Rubin BP, Antonescu CR, Gannon FH et al, Protocol for the examination of specimens from patients with tumors of bone. Arch Pathol Lab Med 2010; 134: e1–e7.
Glasser DB, Lane JM, Huvos AG et al, Survival, prognosis, and therapeutic response in osteogenic sarcoma. The Memorial Hospital experience. Cancer 1992; 69: 698–708.
Juergens H, Kosloff C, Nirenberg A et al, Prognostic factors in the response of primary osteogenic sarcoma to preoperative chemotherapy (high-dose methotrexate with citrovorum factor). Natl Cancer Inst Monogr 1981; 56: 221–226.
Rosenberg AE, Cleton-Jansen AM, de Pinieux G et al, Conventional osteosarcoma. In: Fletcher CDM, Bridge JA, Hogendoorn PC et al, (eds). WHO Classification of Tumours of Soft Tissue and Bone, 4th edn. International Agency for Research on Cancer (IARC): Lyon, 2013, pp 282–288.
Oliveira A, Okada K, Squire J. Telangiectatic osteosarcoma. In: Fletcher CDM, Bridge JA, Hogendoorn PC et al, (eds). WHO Classification of Tumours of Soft Tissue and Bone, 4th edn. International Agency for Reseach on Cancer (IARC): Lyon, 2013, pp 289–290.
Enneking WF, Spanier SS, Goodman MA . A system for the surgical staging of musculoskeletal sarcoma. 1980. Clin Orthop Relat Res 2003; 415: 4–18.
Mirra JM, Picci P, Gold RH . Bone Tumors: Clinical, Radiologic, and Pathologic Correlations. Lea & Febiger: Philadelphia, 1989.
Czerniak B . Dorfman and Czerniak’s Bone Tumors, 2nd edn. Elsevier: Philadelphia, 2016.
Bacci G, Ferrari S, Delepine N et al, Predictive factors of histologic response to primary chemotherapy in osteosarcoma of the extremity: study of 272 patients preoperatively treated with high-dose methotrexate, doxorubicin, and cisplatin. J Clin Oncol 1998; 16: 658–663.
Bacci G, Bertoni F, Longhi A et al, Neoadjuvant chemotherapy for high-grade central osteosarcoma of the extremity: histologic response to preoperative chemotherapy correlates with histologic subtype of the tumor. Cancer 2003; 97: 3068–3075.
Hauben EI, Weeden S, Pringle J et al, Does the histological subtype of high-grade central osteosarcoma influence the response to treatment with chemotherapy and does it affect overall survival? A study on 570 patients of two consecutive trials of the European Osteosarcoma Intergroup. Eur J Cancer 2002; 38: 1218–1225.
Bjornsson J, Inwards CY, Wold LE et al, Prognostic significance of spontaneous tumour necrosis in osteosarcoma. Virchows Arch A Pathol Anat Histopathol 1993; 423: 195–199.
Springfield DS, Schakel ME Jr, Spanier SS . Spontaneous necrosis in osteosarcoma. Clin Orthop Relat Res 1991; 233–237.
Song WS, Jeon DG, Cho WH et al, Spontaneous necrosis and additional tumor necrosis induced by preoperative chemotherapy for osteosarcoma: a case-control study. J Orthop Sci 2015; 20: 174–179.
Robl B, Pauli C, Botter SM et al, Prognostic value of tumor suppressors in osteosarcoma before and after neoadjuvant chemotherapy. BMC Cancer 2015; 15: 379.
Scotlandi K, Serra M, Manara MC et al, Clinical relevance of Ki-67 expression in bone tumors. Cancer 1995; 75: 806–814.
Hernandez-Rodriguez NA, Correa E, Sotelo R et al, Ki-67: a proliferative marker that may predict pulmonary metastases and mortality of primary osteosarcoma. Cancer Detect Prev 2001; 25: 210–215.
Sorensen FB, Jensen K, Vaeth M et al, Immunohistochemical estimates of angiogenesis, proliferative activity, p53 expression, and multiple drug resistance have no prognostic impact in osteosarcoma: a comparative clinicopathological investigation. Sarcoma 2008; 2008: 874075.
Jong R, Davis AM, Mendes MG et al, Proliferative activity (ki-67 expression) and outcome in high grade osteosarcoma: a study of 27 cases. Sarcoma 2000; 4: 47–55.
Molendini L, Benassi MS, Magagnoli G et al, Prognostic significance of cyclin expression in human osteosarcoma. Int J Oncol 1998; 12: 1007–1011.
Price CH . The grading of osteogenic sarcoma. Br J Cancer 1952; 6: 46–68.
Larsson SE, Lorentzon R, Wedren H et al, Osteosarcoma: a multifactorial clinical and histopathological study with special regard to therapy and survival. Acta Orthop Scand 1978; 49: 571–581.
Meister P, Konrad E, Lob G et al, Osteosarcoma: histological evaluation and grading. Arch Orthop Trauma Surg 1979; 94: 91–98.
Campanacci M, Cervellati G . Osteosarcoma: a review of 345 cases. Ital J Orthop Traumatol 1975; 1: 5–22.
Gavanis MB, Whitesides TE Jr . The unreliability of prognostic criteria in osteosarcoma. Am J Clin Pathol 1970; 53: 15–20.
Lewis DR, Gloeckler Ries LA Cancers of the Bone and Joint. In: Gloecker Ries LA, Young JL, Keel GE et al, (eds) SEER Survival Monograph: Cancer Survival Among Adults: U.S. SEER Program, 1988-2001, Patient and Tumor Characteristics. National Cancer Institute, SEER Program: Bethesda, MD, 2007, pp 81–88.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Additional information
Supplementary Information accompanies the paper on Modern Pathology website
Supplementary information
Rights and permissions
About this article
Cite this article
Cates, J., Dupont, W. Cytologic anaplasia is a prognostic factor in osteosarcoma biopsies, but mitotic rate or extent of spontaneous tumor necrosis are not: a critique of the College of American Pathologists Bone Biopsy template. Mod Pathol 30, 52–59 (2017). https://doi.org/10.1038/modpathol.2016.163
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/modpathol.2016.163