Abstract
The oncogenic role of TCL1 in chronic lymphocytic leukemia is well established in transgenic mice. TCL1 expression in other B-cell malignancies has been also described: post-germinal center-derived malignancies, such as multiple myeloma, classically do not express TCL1. Waldenström macroglobulinemia is a post-germinal center malignancy that is known to be similar to chronic lymphocytic leukemia in terms of its gene expression profile. TCL1 expression has not been so far assessed in Waldenström macroglobulinemia. Transcriptomic explorations show that TCL1A expression is linked to signaling pathways and biological functions that are known to be involved in Waldenström macroglobulinemia as well as to gene signatures of interest in B-cell malignancies. We investigated TCL1 expression at the protein level in the bone marrow of a series of 59 patients with Waldenström macroglobulinemia: 76% of patients expressed TCL1, which appeared to be associated with a pejorative prognostic impact. TCL1 could have an oncogenic role in Waldenström macroglobulinemia, and deserves further exploration.
Similar content being viewed by others
Main
Waldenström macroglobulinemia is an uncommon B-cell lymphoproliferative disorder characterized by lymphoplasmacytic cells that infiltrate the bone marrow, along with the presence of an immunoglobulin M monoclonal protein in the serum.1 Waldenström macroglobulinemia is thought to be derived from B cells in which differentiation has been arrested after a somatic hypermutation process in the germinal center and before terminal maturation into plasma cells.2 Oncogenesis may be a result of dysregulation of the NF-κB and PI3K/Akt/mTOR signaling pathways,3, 4, 5 and from the influence of the bone marrow microenvironment.6, 7 Furthermore, whole-genome sequencing of Waldenström macroglobulinemia patients has recently uncovered highly recurring MYD88L265P and WHIM-like CXCR4 mutations that have a potential role in such pathways.8, 9
The T-cell leukemia/lymphoma 1 (TCL1) protein encoded by the TCL1A gene (TCL1) is normally expressed during the early steps of B- and T-cell lymphopoiesis.10 TCL1 has been shown to be overexpressed in chronic lymphocytic leukemia,11, 12 and its oncogenic role has been well established in transgenic mice.13 It acts as an activator of Akt14 and NF-kB15 signaling in the cytoplasm, but may also function as a transcriptional regulator in the nucleus.16 Increased TCL1 expression has been observed in other B-cell disorders17, 18 but not in multiple myeloma. TCL1 expression level still needs to be investigated in Waldenström macroglobulinemia and is the focus of this report.
Materials and methods
Analysis of Transcriptomic Datasets
Transcriptomic data were obtained from native data taken from two publicly available datasets (GSE6691 and GSE12668 datasets). The GSE6691 GEO dataset (56 samples) provided insight into the differences between cells from Waldenström macroglobulinemia and their cell counterparts in chronic lymphocytic leukemia and multiple myeloma. These data were analyzed to compare respective TCL1A expressions in these disease entities using the GEO2R tool from NCBI. The GSE12668 dataset (22 samples) provided high-resolution genomic analysis of Waldenström macroglobulinemia. These data were analyzed to assess TCL1A-co-regulated genes in Waldenström macroglobulinemia. We performed a new normalization procedure to check the intrinsic qualities of the native data: gene expression levels were normalized using the GC Robust Multi-array Average algorithm and flags were computed using MAS5. Quality assessment of the chips was performed using the affyQC-Report R package. Cluster analysis was performed by hierarchical clustering using Spearman's correlation of similarity measure and an average-linkage algorithm. Data were analyzed using QIAGEN’s Ingenuity Pathway Analysis,19 which assessed the relationships between genes and proteins, and constructed pathways to unveil the relevant biological processes.
Patients and Samples
Fifty-nine patients who underwent a bone marrow trephine biopsy for Waldenström macroglobulinemia, at Clermont-Ferrand and La Pitié-Salpêtrière University Hospitals between 1998 and 2012, were included in accordance with the Declaration of Helsinki. All included patients presented with Waldenström macroglobulinemia as defined by the international guidelines according to the World Health Organization classification20 and the Second International Workshop on Waldenström’s Macroglobulinemia,1 ie, bone marrow infiltrated by immunoglobulin M producing clonal lymphoplasmacytic lymphoma. All patients were treated according to the Athens criteria.1 Their characteristics are shown in Table 1. The median follow-up was 103 months (6–261 months).
Immunohistochemistry and Scoring
Analyses were performed in the Clermont-Ferrand pathology laboratory using monoclonal mouse anti-human TCL1 antibody (Clone 27D6/20; MBL International Corporation, Des Plaines, IL, USA; 1/100 dilution for 1 h) and monoclonal mouse anti-human CD20 antibody (Clone L26; Dako, Carpinteria, CA, USA; 1/100 dilution for 1 h). Immunostaining was assessed by three independent investigators who were blinded to the clinical data. If there was disagreement, a second review was conducted.
To assess the tumor infiltrates (stained by hematoxylin and eosin, and by anti-CD20 antibody), the percentage of TCL1-stained tumor cells was determined using a previously published four-tier scoring system for TCL1 assessment in B-cell malignancies,11, 17 as follows: 0=negative; 1=<5% of tumor cells positive; 2=positive in up to 50% of tumor cells; and 3=positive in >50% of tumor cells. TCL1 staining intensity was also recorded using a previously published three-tier score (0=no staining; 1=low intensity; 2=high intensity)11 to evaluate the intra-tumoral distribution and level of TCL1 expression within each tumor sample.
Statistical Analyses
Therapeutic requirements and the time until endpoints were defined according to published recommendations.1, 21, 22 Pearson Chi-square test, two-sided Fisher test, Mann–Whitney test, Kaplan–Meier method, and log-rank test were applied to the data in appropriate settings, using SPSS Statistics v22 (IBM), PRISM v6.0 (Graphpad), and/or R23 software.
Results
TCL1A Expression and Related Transcriptome
To investigate the potential role of TCL1 in the biology of Waldenström macroglobulinemia, we first analyzed publicly available transcriptomic datasets. Analyses of data from GSE6691 showed that TCL1A mRNA expression levels of Waldenström macroglobulinemia cells ranged from those observed in multiple myeloma to those observed in chronic lymphocytic leukemia (P<0.001) (Figure 1a). In order to analyze TCL1-related transcriptome, we defined two groups of patients according to TCL1A expression using the GSE12668 dataset: ie, a TCL1Alow group with normalized fluorescence of 285 (<25th percentile) and a TCL1Ahigh group with normalized fluorescence of 3186 (>75th percentile).
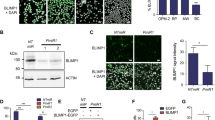
TCL1A transcriptomic expression: comparative exploration and biological relevance. (a) TCL1A transcriptomic expression was compared within three different B-cell malignancies: chronic lymphocytic leukemia (CLL), multiple myeloma (MM), and Waldenström macroglobulinemia (WM). TCL1A transcription level in WM was intermediate between CLL and MM, as was expected regarding the classical precocious post-germinal center normal counterpart in WM. (b) Main canonical pathways were positively linked to a high TCL1A transcription level. In order to show only the strongest and most reliable data, we show all pathways applicable to hematological malignancies that had a –log(P-value) >5 and were linked to biological function. It is noteworthy that the main pathways implicated in the pathophysiology of WM are among the canonical pathways linked to TCL1A's high transcription level (BCR, PI3K/Akt, mTOR, and ATM). (c) TCL1A could be directly (full line) or indirectly (dotted line) linked to the main biological actors in WM (actors significantly more expressed in TCL1Ahigh cells are colored (red or light grey)). Of note, in this study, MYD88 and TCL1 transcription levels were correlated (MYD88 ratio 1.23; delta: 142; P=0.024). A full color version of this figure is available at the Modern Pathology journal online.
We checked that median TCL1A expression in the TCL1Alow group was 10-fold higher than the background noise and that TCL1A expression was significantly different between both groups (mean TCL1Ahigh/TCL1Alow ratio of 11, and a mean delta (TCL1Ahigh – TCL1Alow) of 2901) (P<0.001). We then compared the gene expression profile related to each group (TCL1Ahigh vs TCL1Alow).
Consistent with the oncogenic role of TCL1, most relevant signaling pathways associated with TCL1Ahigh expression are known to be involved in cell cycle, apoptosis, and the DNA-damage response. Interestingly, TCL1Ahigh expression was associated with signaling pathways previously shown to be relevant in Waldenström macroglobulinemia (ie, PI3K/AKT, mTOR, BCR signal3, 4, 5) (Figure 1b). The highlighted biological functions related to TCL1A agreed with these results (Supplementary Table 1). In addition, TCL1Ahigh was associated with gene signatures of interest in tumor B-cell progression or resistance (eg, activated MYC, CCND1, SOX11 signatures, and an inhibited TP53 signature) (Supplementary Table 2).
By computing these data and using published data on TCL1A, we explored the putative interactions of TCL1A in Waldenström macroglobulinemia tumor cells. TCL1A could be linked to crucial actors in the pathophysiology of Waldenström macroglobulinemia within a tightly coupled network (Figure 1c).
Impact of TCL1 Protein Expression on Survival
Immunostaining of bone marrow tumor cells revealed that TCL1 was expressed at the protein level in 45 (76%) Waldenström macroglobulinemia patients. This expression was weak in 2, moderate in 17, and strong in 26 patients (Figure 2a). TCL1 was preferentially detected in the cytoplasm compared with the nucleus.
TCL1 protein expression: characteristics and clinical impacts on the time to next treatment. (a) TCL1 protein expression was assessed with a previously published four-tier scoring system that focused on the percentage of CD20+ tumor cells positive for TCL1 staining. Most patients showed 'moderate to strong' expression of TCL1. (b) Patients with moderate/strong TCL1 expression had a shorter time to next treatment compared with those with negative/weak expression (respectively 16.4 vs 59.4 months, HR 2.07 (1.037–3.692), P=0.036).
Regarding the clinical relevance of TCL1 expression in Waldenström macroglobulinemia, we failed to demonstrate any significant correlation between TCL1 expression pattern and the Waldenström macroglobulinemia features known to affect the prognosis (Table 1). However, patients with moderate/strong TCL1 expression had a significantly shorter time to next treatment compared with patients with negative/weak expression (16.4 vs 59.4 months, respectively; HR 2.07 (95% CI: 1.037–3.692), P=0.036) (Figure 2b). No significant impact was observed in terms of progression-free survival or overall survival.
Discussion
Herein, for the first time, we have highlighted the expression of the TCL1 oncogene in Waldenström macroglobulinemia. The TCL1 transcriptomic targets were in line with the biology of Waldenström macroglobulinemia, and high TCL1 expression was associated with a poor outcome.
Using analyses from transcriptomic datasets, we found that TCL1 expression levels ranged from those observed in multiple myeloma to those observed in chronic lymphocytic leukemia. Moreover, gene expression profiles related to TCL1 gene expression were similar to the gene signatures of interest in B-cell malignancies, and corresponded to the biological pathways involved in cell cycle, apoptosis, and the DNA-damage response, which are known to be implicated in Waldenström macroglobulinemia oncogenesis. Altogether, these results suggest that TCL1 could have an oncogenic role in Waldenström macroglobulinemia.
Most patients expressed TCL1 at a protein level. Moreover, the high percentage of tumor cells that expressed TCL1 was linked to a poor outcome but was not associated with classical agressive features. Interestingly, staining intensity was heterogeneous within the tumor infiltrates, as has been described in chronic lymphocytic leukemia,11 which questions the mechanisms underlying TCL1 overexpression in Waldenström macroglobulinemia.
TCL1 was preferentially detected in the cytoplasm compared with the nucleus: this has also been observed in Burkitt's lymphoma, but both cytoplasmic and nuclear localization were then seen in pre-germinal center B-cell lymphoma.17
On the basis of these observations, two major issues have to be addressed. First, the published data indicate that TCL1 is not expressed in patients with marginal-zone lymphoma.17, 24 Differential diagnosis of Waldenström macroglobulinemia with marginal-zone lymphoma can be challenging, and its diagnosis directly impacts on the choice of therapeutic options. Our data suggest that TCL1 expression could be a differential marker between Waldenström macroglobulinemia and marginal-zone lymphoma, although this remains to be explored further. In addition, if TCL1 expression can clearly distinguish Waldenström macroglobulinemia from other B-cell malignancies, including marginal-zone lymphoma and multiple myeloma, such a difference, as demonstrated by the MYD88L265P mutation, could provide important insights regarding the specific patterns of tumor development in Waldenström macroglobulinemia.
Overall, our results indicate that the TCL1 oncogene is significantly expressed in most of Waldenström macroglobulinemia cases, in which it could drive important pathways and adversely impact on prognosis. Added value of TCL1 staining for Waldenström macroglobulinemia diagnosis remains to be investigated.
References
Owen RG, Treon SP, Al-Katib A et al. Clinicopathological definition of Waldenstrom’s macroglobulinemia: consensus panel recommendations from the Second International Workshop on Waldenstrom’s Macroglobulinemia. Semin Oncol 2003;30:110–115.
Sahota SS, Forconi F, Ottensmeier CH et al. Origins of the malignant clone in typical Waldenstrom’s macroglobulinemia. Semin Oncol 2003;30:136–141.
Leleu X, Jia X, Runnels J et al. The Akt pathway regulates survival and homing in Waldenstrom macroglobulinemia. Blood 2007;110:4417–4426.
Leleu X, Eeckhoute J, Jia X et al. Targeting NF-kappaB in Waldenstrom macroglobulinemia. Blood 2008;111:5068–5077.
Roccaro AM, Sacco A, Husu EN et al. Dual targeting of the PI3K/Akt/mTOR pathway as an antitumor strategy in Waldenstrom macroglobulinemia. Blood 2010;115:559–569.
Elsawa SF, Novak AJ, Ziesmer SC et al. Comprehensive analysis of tumor microenvironment cytokines in Waldenstrom macroglobulinemia identifies CCL5 as a novel modulator of IL-6 activity. Blood 2011;118:5540–5549.
Hodge LS, Ziesmer SC, Yang ZZ et al. IL-21 in the bone marrow microenvironment contributes to IgM secretion and proliferation of malignant cells in Waldenstrom macroglobulinemia. Blood 2012;120:3774–3782.
Treon SP, Xu L, Yang G et al. MYD88 L265P somatic mutation in Waldenström’s macroglobulinemia. N Engl J Med 2012;367:826–833.
Hunter ZR, Xu L, Yang G et al. The genomic landscape of Waldenstrom macroglobulinemia is characterized by highly recurring MYD88 and WHIM-like CXCR4 mutations, and small somatic deletions associated with B-cell lymphomagenesis. Blood 2014;123:1637–1646.
Teitell MA . The TCL1 family of oncoproteins: co-activators of transformation. Nat Rev Cancer 2005;5:640–648.
Herling M, Patel KA, Khalili J et al. TCL1 shows a regulated expression pattern in chronic lymphocytic leukemia that correlates with molecular subtypes and proliferative state. Leukemia 2006;20:280–285.
Herling M, Patel KA, Weit N et al. High TCL1 levels are a marker of B-cell receptor pathway responsiveness and adverse outcome in chronic lymphocytic leukemia. Blood 2009;114:4675–4686.
Bichi R, Shinton SA, Martin ES et al. Human chronic lymphocytic leukemia modeled in mouse by targeted TCL1 expression. Proc Natl Acad Sci USA 2002;99:6955–6960.
Laine J, Künstle G, Obata T et al. The protooncogene TCL1 is an Akt kinase coactivator. Mol Cell 2000;6:395–407.
Gaudio E, Spizzo R, Paduano F et al. Tcl1 interacts with Atm and enhances NF-κB activation in hematologic malignancies. Blood 2012;119:180–187.
Pekarsky Y, Palamarchuk A, Maximov V et al. Tcl1 functions as a transcriptional regulator and is directly involved in the pathogenesis of CLL. Proc Natl Acad Sci USA 2008;105:19643–8.
Herling M, Patel KA, Hsi ED et al. TCL1 in B-cell tumors retains its normal b-cell pattern of regulation and is a marker of differentiation stage. Am J Surg Pathol 2007;31:1123–1129.
Aggarwal M, Villuendas R, Gomez G et al. TCL1A expression delineates biological and clinical variability in B-cell lymphoma. Mod Pathol 2009;22:206–215.
QIAGEN Redwood City. IPA. Available on: http://www.qiagen.com/ingenuity.
Swerdlow SH, Berger F, Pileri SA et al. Lymphoplasmacytic lymphoma. In: Swerdlow SH, Campo E, Harris NL et al. (eds). WHO Classification of Tumours of Haematopoietic and Lymphoid Tissues. 4th edn. Lyon, France: WHO/IARC: Lyon, France, 2008, pp 194–195.
Owen RG, Kyle RA, Stone MJ et al. Response assessment in Waldenström macroglobulinaemia: update from the VIth International Workshop. Br J Haematol 2013;160:171–176.
Rourke M, Anderson KC, Ghobrial IM . Review of clinical trials conducted in Waldenstrom macroglobulinemia and recommendations for reporting clinical trial responses in these patients. Leuk Lymphoma 2010;51:1779–1792.
R Core Team. R: a language and environment for statistical computing [Internet]. R Foundation for Statistical Computing: Vienna, Austria, 2014, Available on: http://www.R-project.org.
Narducci MG, Pescarmona E, Lazzeri C et al. Regulation of TCL1 expression in B- and T-cell lymphomas and reactive lymphoid tissues. Cancer Res 2000;60:2095–2100.
Acknowledgements
We acknowledge Laetitia Pere and Emmanuel Bourgeois from the Clermont-Ferrand pathology laboratory for their technical and logistical assistance.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interests.
Additional information
Supplementary Information accompanies the paper on Modern Pathology website
Supplementary information
Rights and permissions
About this article
Cite this article
Lemal, R., Bard-Sorel, S., Montrieul, L. et al. TCL1 expression patterns in Waldenström macroglobulinemia. Mod Pathol 29, 83–88 (2016). https://doi.org/10.1038/modpathol.2015.122
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/modpathol.2015.122
This article is cited by
-
Two high-risk susceptibility loci at 6p25.3 and 14q32.13 for Waldenström macroglobulinemia
Nature Communications (2018)
-
Transgenic mouse model of IgM+ lymphoproliferative disease mimicking Waldenström macroglobulinemia
Blood Cancer Journal (2016)