Abstract
Follicular lymphoma is characterized by aberrant BCL2 expression, a feature that is exploited for diagnostic purposes. However, a certain percentage of follicular lymphomas might be BCL2-negative by immunohistochemistry, increasing the difficulties in differentiating them from follicular hyperplasia. The expression of TUBB3 has been recently reported as negative in a small series of follicular lymphomas. We have therefore tested a larger series, including 61 BCL2-positive and 25 BCL2-negative cases, and compared them with 61 reactive lymphoid tissues. First, a subjective score of TUBB3 staining was applied, showing that it was consistently positive in reactive germinal centers, while most follicular lymphomas were negative; in fact, only 10/61 (16%) BCL2-positive and 1/25 (4%) BCL2-negative cases showed a positive staining for TUBB3, while 58/61 (95%) of tissues with follicular hyperplasia were positive. The application of a standardized scoring system to a large number of follicles, based on virtual slides, demonstrated that reactive lymphoid tissues had a significantly higher number of TUBB3-positive follicles both compared with BCL2-positive cases and to BCL2-negative cases. Our data support the use of TUBB3 staining in differentiating follicular lymphoma, including BCL2-negative cases, from follicular hyperplasia.
Similar content being viewed by others
Main
Follicular lymphoma is one of the commonest lymphomas in the western world.1 It is characterized by a neoplastic proliferation of lymphocytes that are organized in a follicular pattern in the majority of cases. The distinction between follicular lymphoma and follicular hyperplasia can be challenging, and immunohistochemistry is often used to help this distinction. The most helpful markers for this purpose are Ki-67 (usually showing a lower proliferation index in follicular lymphoma) and BCL2, which is usually positive in follicular lymphoma due to constitutive expression as a result of the t(14;18) translocation; on the contrary, the BCL2 protein is downregulated in reactive germinal centers (a physiological mechanism favoring apoptosis in this environment). However, up to 10% of follicular lymphomas can be BCL2-negative by immunohistochemistry,2 in part due to mutations in the antibody-binding site.3 The use of new antibodies can increase the rate of BCL2 staining,4 but nevertheless there is a core of really BCL2-negative follicular lymphomas, often lacking the typical t(14;18) translocation.5 In these cases, the differential diagnosis relies only on morphological grounds and on the lower proliferation index. However, this latter parameter can be slippery, because sectioning artifacts in follicular hyperplasia can show only parts of follicles with a lower proliferation, while BCL2-negative follicular lymphoma can sometimes have a high proliferation index. Problems may therefore arise in the differential diagnosis between follicular lymphoma (especially in BCL2-negative cases) and follicular hyperplasia.
TUBB3 is an isotype of beta-tubulin that has been described as normally expressed in the central nervous system,6 in vascular endotelia7 and, more recently, in follicular dendritic cells.8 TUBB3 differs from other tubulins in the fact that its mutation does not impair cortical cell migration but impairs axon guidance and maintenance.9 Its precise function in non-neuronal cells is almost unknown.10 Its gene resides on 16q24.3, a region frequently altered in lymphoma.11 TUBB3 expression has been correlated with poor response to some chemotherapeutic drugs, such as taxanes and vinca alkaloids.12, 13
A recent study has analyzed TUBB3 expression in several lymphoma subtypes;14 among B-cell lymphomas, TUBB3 expression was shown to be limited to the majority of Burkitt lymphomas and a subset of about 15% of diffuse large B-cell lymphomas. A series of 12 follicular lymphomas (presumably BCL2-positive) was also tested, which was reported as completely negative.
On these grounds, we hypothesized that TUBB3 staining might be helpful in the distinction between follicular lymphoma and follicular hyperplasia. We therefore tested the presence of TUBB3 by immunohistochemistry in a larger series, including both BCL2-postive and BCL2-negative follicular lymphoma cases, as well as several reactive lymphoid tissues showing features of follicular hyperplasia. Our data support the use of TUBB3 in the differential diagnosis between follicular lymphoma (both BCL2-positive and BCL2-negative) and follicular hyperplasia.
Materials and methods
Cases
A retrospective database search retrieved 25 BCL2-negative follicular lymphoma cases in the time frame 1999–2012. Sixty-one BCL2-positive follicular lymphoma were retrieved in the time frame 2009–2012. Cases with a purely diffuse pattern were excluded from the study as statistical follicle counting would not have been possible. Sixty-one reactive tissues with an abundant reactive follicular component were selected as controls, including 20 lymph nodes, 38 tonsils, 1 spleen and 2 appendices. Thirteen nodal marginal zone lymphomas showing features of follicular colonization were also evaluated.
Immunohistochemistry
Immunohistochemical staining was performed using previously described reagents and protocols.15 Anti-TUBB3 antibody (clone TUJ1, Covance, Princeton, NJ, USA) was diluted 1/500 and a 95°X15’ER2 pH8-9 was used for antigen retrieval. To exclude false-negative BCL2 staining, two different antibody clones were used (clone 124, Dako and clone EP36, Epitomics).4 Detection was performed by a polymer-based system (Bond Polymer Refine Detection, Leica Biosystems, Nussloch, Germany) on an automated stainer (Leica Bond-Max).
Standardized Follicle Scoring
Subjective scoring was obtained by defining score values as depicted in Supplementary Figure 1. Scores were determined as 0 for a totally negative case (with internal control), 1h (heterogeneous) if <20% follicles showed a reliable staining, 1d (diffuse) if all or most follicles showed a very light staining, difficult to distinguish from background, 2h if 20–80% of follicles showed a strong staining, 2d when most or all follicles showed an intermediate but unmistakably positive staining, 3 when all follicles were strongly positive. For practical purposes, 0 and 1 scores were considered negative, and 2 and 3 as positive. In the subjective scoring procedures, follicles considered to be reactive (polarized, polymorphic, with high proliferation index and/or BCL2-negative) were not considered. In order to obtain reliable results for statistical purposes, we also adopted a more rigorous counting and scoring approach. This involved the preparation of a virtual slide by using the D-sight 200 device (Menarini Diagnostics, Florence, Italy) and then counting and scoring each follicle on the slide (see Supplementary Figure 2 for examples).
Statistics
A Monte Carlo permutation approach was used to assess the significance of the difference of TUBB3-positive follicle frequency between groups. The group label (follicular hyperplasia, follicular lymphoma-BCL2 negative, follicular lymphoma-BCL2 positive) was randomly permuted among samples, and the ratio of positive follicle frequency values (simulated-ratio) between the tested groups was computed. The procedure was repeated 1 000 000 times. Significance of positive follicle frequency differences was estimated by counting the number of times (over a total of 1 000 000) in which the simulated-ratio value was equal or greater than the positive follicle frequency ratio estimated in the original sample.
For other features, Fisher’s exact test (two-tailed) was used to estimate the significance of differences between BCL2-positive follicular lymphoma and BCL2-negative follicular lymphoma.
Results
The features of BCL2-positive and BCL2-negative cases are reported in Table 1 (the complete data set is available as Supplementary Table S1). The two sets did not differ significantly for most markers, except for a statistically significant (P=0.0011) difference in the 2h pattern of TCL1A staining and a statistically significant (P=0.0043) difference in the age of the patients in each group. There was also a significantly higher number of CD10-negative cases (P=0.047) among BCL2-negative follicular lymphomas.
Overall, after subjective standardized scoring, 13% (11/86) of all follicular lymphomas were positive (scores 2 and 3) for TUBB3; namely, 10/61 (16%) of BCL2-positive and 1/25 (4%) of BCL2-negative cases (Table 1 and Figure 1). Among reactive tissues, only 3/61 (5%) were scored as 1h, whereas 58/61 (95%) were scored as 2h or 3. The results of subjective and virtual slide counting and scoring are summarized in Supplementary Table S1. A total of 9655 follicles were counted for BCL2-positive follicular lymphomas (average 158, range 17–616), 3045 for BCL2-negative follicular lymphomas (average 122, range 20–310) and 6256 for reactive samples (average 102, range 8–464). There was a statistically significant difference in the number of positive follicles between all follicular lymphomas (BCL2-positive and BCL2-negative) against follicular hyperplasia (P<0.000001), between BCL2-positive follicular lymphomas and follicular hyperplasia (P<0.000001), BCL2-negative follicular lymphomas and follicular hyperplasia (P<0.000001) but not between BCL2-positive and BCL2-negative follicular lymphomas (P=0.460706; Table 2 and Figure 2).
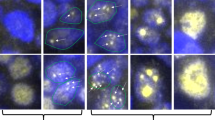
Expression of TUBB3 in BCL2-positive follicular lymphoma, BCL2-negative follicular lymphoma and follicular hyperplasia. Left column: BCL2-positive follicular lymphoma; center column: BCL2-negative follicular lymphoma; right column: follicular hyperplasia. (a–c): H&E staining; (d–f): BCL2 staining; (g–i): TCL1A staining; and (j–l): TUBB3 staining.
All nodal marginal zone lymphomas were completely negative for TUBB3 staining (0/13, 0%).
Discussion
The diagnosis of follicular lymphoma is usually straightforward; nevertheless a number of cases might be difficult to differentiate from follicular hyperplasia, especially when BCL2 is undetectable by immunohistochemistry and/or the proliferation index is relatively high. A group of cases are truly BCL2-negative and usually lack the t(14;18) translocation.4 These cases are probably biologically different from classical follicular lymphoma5 and their pathological features are less solid than those of BCL2-positive follicular lymphoma. Novel markers that might help in the discrimination between follicular hyperplasia and BCL2-negative follicular lymphoma might therefore be useful, especially in a non-expert environment where a pathologist might feel uncomfortable in diagnosing BCL2-negative follicular lymphoma.
In our study, we have investigated the expression of TUBB3, a beta-tubulin isotype that seems to have non-redundant functions compared with other tubulins,10 in follicular lymphoma; our series included both BCL2-positive and BCL2-negative cases, as well as a series of control tissues. Our series is therefore the first to investigate TUBB3 in BCL2-negative cases and the largest overall considering both the total number of follicular lymphomas and of reactive samples. Moreover, we applied a standardized scoring system not used by previous investigators.
Our data show that TUBB3 is widely expressed in germinal center centrocytes and centroblasts of 95% of cases of follicular hyperplasia, at variance with a previous study, using the same antibody, reporting expression only in follicular dendritic cells.8 This expression in reactive germinal centers is particularly important, because we found that the large majority of follicular lymphomas are TUBB3 negative, both in the BCL2-positive and in the BCL2-negative subsets. The occasional expression of TUBB3 was often associated with residual non-neoplastic germinal centers in the sample, as demonstrated by the high Ki-67 index and the absence of BCL2 expression in a few cases with a heterogeneous pattern of staining. The recognition of non-neoplastic follicles in follicular lymphoma has been demonstrated to confer a better prognosis,16 so TUBB3 might help in this regard.
Being a microtubule protein, it might be hypothesized that the low expression of TUBB3 reflects the low proliferation index of follicular lymphoma. However, we could not correlate the degree of TUBB3 expression with the Ki-67 index, and indeed a few cases with relatively high Ki-67 were consistently TUBB3 negative.
We found 11/86 (13%) follicular lymphomas positive for TUBB3; their immunohistochemical pattern, grading, sex and age do not differ statistically from the TUBB3-negative follicular lymphomas. However, we noticed that only 1/11 cases (9%) was BCL2 negative whereas 10/11 (91%) were BCL2 positive. This difference was not significant, but this might be due to the low number of cases analyzed. The very rare expression of TUBB3 in BCL2-negative follicular lymphoma makes it even more attractive as a marker in a diagnostic environment.
In a lot of cancers such as non-small cell lung carcinoma, breast carcinoma, ovarian carcinoma, high expression of TUBB3 was reported to confer resistance to vinca alkaloids and taxanes therapy.17, 18, 19 It would therefore be interesting to investigate whether TUBB3-positve cases behave differently from the negative majority from a therapeutical point of view. However, considering that the majority of cases are negative, a very large series should be investigated.
Although not primarily the aim of this work, we confirm the usefulness of TCL1A as a diagnostic aid in BCL2-negative follicular lymphoma, a feature possibly due to differential microRNA expression.20 Indeed, we found a significantly higher number of cases displaying an heterogeneous pattern in BCL2-negative follicular lymphoma, which might be a diagnostic clue, as follicular hyperplasia is usually uniformly TCL1A positive.
A small series of 13 nodal marginal zone lymphomas with features of follicular colonization, which might mimic follicular lymphoma, was investigated. All tested cases resulted negative for TUBB3, therefore we conclude that this marker is not helpful in distinguishing nodal marginal zone lymphomas from follicular lymphoma.
In conclusion, we have investigated the expression of TUBB3 in several reactive lymphoid tissues and follicular lymphomas. Our data show that normal germinal center lymphocytes express TUBB3, whereas the large majority of follicular lymphomas, including BCL2-negative follicular lymphomas, do not. Although the biological significance of this finding remains unknown, it can be usefully applied to the routine diagnostic procedures when a differential diagnosis of follicular lymphoma vs follicular hyperplasia is considered.
References
Harris NL, Swerdlow SH, Jaffe ES et al. Follicular lymphoma In: Swerdlow SH, Campo E, Harris NL, et al (eds). WHO Classification of Tumours of Haematopoietic and Lymhpoid Tissues. IARC Press: Lyon, France, 2008, pp 220–226.
Lai R, Arber DA, Chang KL et al. Frequency of bcl-2 expression in non-Hodgkin's lymphoma: a study of 778 cases with comparison of marginal zone lymphoma and monocytoid B-cell hyperplasia. Mod Pathol 1998;11:864–869.
Schraders M, de Jong D, Kluin P et al. Lack of Bcl-2 expression in follicular lymphoma may be caused by mutations in the BCL2 gene or by absence of the t(14;18) translocation. J Pathol 2005;205:329–335.
Masir N, Campbell LJ, Goff LK et al. BCL2 protein expression in follicular lymphomas with t(14;18) chromosomal translocations. Br J Haematol 2009;144:716–725.
Leich E, Salaverria I, Bea S et al. Follicular lymphomas with and without translocation t(14;18) differ in gene expression profiles and genetic alterations. Blood 2009;114:826–834.
Katsetos CD, Herman MM, Mork SJ . Class III beta-tubulin in human development and cancer. Cell Motil Cytoskeleton 2003;55:77–96.
Kang J, Lee I . TuJ1 (class III β-tubulin) as phenotypic marker of lymphatic and venous valves. Cardiovasc Pathol 2006;15:218–221.
Lee S, Choi K, Ahn H et al. TuJ1 (class III beta-tubulin) expression suggests dynamic redistribution of follicular dendritic cells in lymphoid tissue. Eur J Cell Biol 2005;84:453–459.
Tischfield MA, Engle EC . Distinct alpha- and beta-tubulin isotypes are required for the positioning, differentiation and survival of neurons: new support for the 'multi-tubulin' hypothesis. Biosci Rep 2010;30:319–330.
Shibazaki M, Maesawa C, Akasaka K et al. Transcriptional and post-transcriptional regulation of betaIII-tubulin protein expression in relation with cell cycle-dependent regulation of tumor cells. Int J Oncol 2012;40:695–702.
Salaverria I, Akasaka T, Gesk S et al. The CBFA2T3/ACSF3 locus is recurrently involved in IGH chromosomal translocation t(14;16)(q32;q24) in pediatric B-cell lymphoma with germinal center phenotype. Genes Chromosomes Cancer 2012;51:338–343.
Ferrandina G, Zannoni GF, Martinelli E et al. Class III beta-tubulin overexpression is a marker of poor clinical outcome in advanced ovarian cancer patients. Clin Cancer Res 2006;12:2774–2779.
Seve P, Lai R, Ding K et al. Class III beta-tubulin expression and benefit from adjuvant cisplatin/vinorelbine chemotherapy in operable non-small cell lung cancer: analysis of NCIC JBR.10. Clin Cancer Res 2007;13:994–999.
Yoon SO, Kim WY, Go H et al. Class III beta-tubulin shows unique expression patterns in a variety of neoplastic and non-neoplastic lymphoproliferative disorders. Am J Surg Pathol 2010;34:645–655.
Munari E, Rinaldi M, Ambrosetti A et al. Absence of TCL1A expression is a useful diagnostic feature in splenic marginal zone lymphoma. Virchows Arch 2012;461:677–685.
Adam P, Schoof J, Hartmann M et al. Cell migration patterns and ongoing somatic mutations in the progression of follicular lymphoma. Cytogenet Genome Res 2007;118:328–336.
Zhang HL, Ruan L, Zheng LM et al. Association between class III beta-tubulin expression and response to paclitaxel/vinorebine-based chemotherapy for non-small cell lung cancer: a meta-analysis. Lung Cancer 2012;77:9–15.
Cittelly DM, Dimitrova I, Howe EN et al. Restoration of miR-200c to ovarian cancer reduces tumor burden and increases sensitivity to paclitaxel. Mol Cancer Ther 2012;11:2556–2565.
Lobert S, Jefferson B, Morris K . Regulation of beta-tubulin isotypes by micro-RNA 100 in MCF7 breast cancer cells. Cytoskeleton (Hoboken) 2011;68:355–362.
Leich E, Zamo A, Horn H et al. MicroRNA profiles of t(14;18)-negative follicular lymphoma support a late germinal center B-cell phenotype. Blood 2011;118:5550–5558.
Acknowledgements
This was supported in part by AIRC (MFAG Grant to AZ), AIRC and Fondazione Cariverona (Regional Grant to MC) and in part by intramural funds of the University of Verona, Verona, Italy.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Additional information
Supplementary Information accompanies the paper on Modern Pathology website
Supplementary information
Rights and permissions
About this article
Cite this article
Zamò, A., Erdini, F., Malerba, G. et al. Lack of expression of TUBB3 characterizes both BCL2-positive and BCL2-negative follicular lymphoma. Mod Pathol 27, 808–813 (2014). https://doi.org/10.1038/modpathol.2013.182
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/modpathol.2013.182