Abstract
Appendiceal mucinous neoplasms have been the focus of considerable debate in recent years. We histologically classified 70 appendiceal mucinous neoplasms into three categories: 32 mucinous adenoma, 23 mucinous neoplasm of uncertain malignant potential, and 15 mucinous adenocarcinomas. Immunohistochemistry was performed for 24 proteins in different functional categories, specifically, oncogenic proteins (bcl-2, β-catenin, CEA, C-erbB2, c-kit, Cox-2, Cyclin D1, EGFR, Ki-67, NF-κB, VEGF), tumor suppressors (E-cadherin, FHIT, hMLH1, p53, p63, smad4), cell-cycle regulators (p21, p27, p16), and mucin proteins (MUC1, MUC2, MUC5AC, MUC6). Our data showed that 9 out of the 24 proteins were more frequently altered in the mucinous adenocarcinoma group than in the mucinous adenoma group (P<0.05), including β-catenin (13% in mucinous adenoma vs 60% in mucinous adenocarcinoma), CyclinD1 (44 vs 87%), Ki-67 (high labeling index: 31 vs 67%), NF-κB (19 vs 60%), VEGF (16 vs 87%), E-cadherin (0 vs 47%), p53 (6 vs 40%), MUC2 (9 vs 67%), and MUC5AC (3 vs 40%). The distinct immunoexpression profile of mucinous neoplasm of uncertain malignant potential was placed between those of mucinous adenoma and mucinous adenocarcinoma (P<0.05). Moreover, the mucinous adenoma, mucinous neoplasm of uncertain malignant potential, and mucinous adenocarcinoma categories displayed differences in terms of the number of altered markers among the nine proteins (P<0.05; mean 1.4 vs 2.6 vs 5.5, respectively). In mucinous adenocarcinoma, the p53 status was related to disease-free survival and overall survival of patients (P<0.05, both). NF-κB status and the number of altered protein markers made statistically marginal impacts on disease-free survival; also β-catenin loss, on overall survival of patients. In conclusion, protein immunoexpression profiles may facilitate the classification of appendiceal mucinous neoplasms. In our study, the three tumor categories of mucinous adenoma, mucinous neoplasm of uncertain malignant potential, and mucinous adenocarcinoma exhibited distinct immunoexpression profiles. Five and more altered protein markers, p53 overexpression, NF-κB positivity, and β-catenin loss were predictive factors of adverse clinical outcomes in appendiceal mucinous adenocarcinomas.
Similar content being viewed by others
Main
Appendiceal mucinous neoplasms are rare tumors, and their diagnostic classification is currently controversial.1, 2, 3, 4, 5, 6, 7, 8, 9, 10, 11, 12 Generally, in appendiceal tumors, the appendiceal wall is totally fibrotic, thus each layer in the wall cannot be discerned. Consequently, it is difficult to establish the tumor invasion status. In practice, the invasion front of appendiceal mucinous adenocarcinomas tends to display a broad pushing feature rather than an infiltrative pattern, imitating its benign counterpart. In addition, even tumors with bland-looking (mucinous adenoma-mimicking) cytoarchitecture can involve other organs, such as ovary and spleen, as well as peritoneum (pseudomyxoma peritonei), and may behave in an aggressive manner.
In 1995, appendiceal mucinous neoplasm of uncertain malignant potential was introduced as a diagnostic entity.2 Mucinous neoplasm of uncertain malignant potential was defined in detail, not to use mucinous neoplasm of uncertain malignant potential as the diagnostic term excessively. In 2005, the term ‘mucinous neoplasm of uncertain malignant potential’ was revisited by Pai et al4 Under conditions where the muscularis mucosae was lost and invasion was unclear, appendiceal mucinous neoplasm displaying bland cytoarchitecture, but features of (1) epithelium pushing deeply into underlying tissue, (2) cystic gland-like structure in wall, or (3) uncertainty of complete excision, such as positive proximal margin or extensive mucin on the appendiceal serosa, were classified as mucinous neoplasm of uncertain malignant potential.4 However, the issue of whether mucinous neoplasm of uncertain malignant potential progresses toward mucinous adenoma or mucinous adenocarcinoma in terms of biologic behavior remains to be established. A number of distinguished researchers have classified appendiceal mucinous neoplasm into two categories, specifically, low-grade appendiceal mucinous neoplasm or mucinous adenocarcinoma.3
Molecular changes, such as protein alterations, which may correspond with the biologic behavior of tumors, are helpful in classifying tumors and predicting clinical outcome. Following examination of the existing literature for immunoexpression data on proteins in mucinous neoplasms of various organs, including colorectum,13, 14, 15, 16, 17, 18 pancreas,19, 20 ovary,21, 22, 23 and appendix,24, 25, 26 we focused on 24 proteins. The immunoexpression profiles of different functional proteins in three groups—mucinous adenoma, mucinous neoplasm of uncertain malignant potential, and mucinous adenocarcinoma of the appendix—were analyzed. Additionally, the clinical implications of immunoexpression patterns were evaluated.
Materials and methods
Patient Samples
We retrieved database information on appendiceal mucinous neoplasms, which were resected at three affiliated hospitals to Seoul National University College of Medicine. In total, 70 cases were included in the study, of which 28 were resected at Seoul National University Hospital from 2003 to 2007, 25 at Seoul National University Boramae Hospital from 1998 to 2007, and 17 at Bundang Seoul National University Hospital from 2003 to 2007. Clinical information, including age, sex, survival data, metastasis to distant organs, recurrence, poor response to treatment or tumor-associated death, was obtained from medical records. The follow-up period was 12–60 months in malignant cases, and 7–120 months in the remaining cases. The histology of all cases was reviewed by two pathologists (MSC and SOY).
Tissue Array
Two to five different representative areas per case were selected. Each tissue core (2.0 mm in diameter) was punched out from the original paraffin block of tumor samples using a trephine apparatus. The punched cores were arranged in a new tissue array paraffin block, containing 59 tissue cores and 1 ink core as a direction indicator. Five tissue array blocks (300 cores) were newly manufactured, which included 70 cases of appendiceal mucinous neoplasms and 32 nonneoplastic appendiceal tissues as a control.
Immunohistochemistry
Immunohistochemistry was performed on 4 μm thick paraffin-embedded tissue sections using the streptavidin–biotin peroxidase complex method after microwave or autoclave-based antigen retrieval. We selected 24 antibodies for immunostaining (Table 1), specific for oncogenic proteins (bcl-2, β-catenin, CEA, C-erbB2, c-kit, Cox-2, Cyclin D1, EGFR, Ki-67, NF-κB, VEGF), tumor suppressors (E-cadherin, FHIT, hMLH1, p53, p63, smad4), cell-cycle regulators (p21, p27, p16), and mucins (MUC1, MUC2, MUC5AC, MUC6). Immunohistochemistry was performed for the 24 proteins after a test procedure using human control slides for immunostaining (Superbiochips Laboratories, Seoul, Korea).
Data from immunohistochemical staining are presented in Table 2. In cases where more than 10% of tumor cells are immunoreactive, immunostaining is regarded as positive27, 28 or high labeling index for Ki-67.29 MUC1, MUC2, MUC5AC, and MUC6 protein expression status is positive when more than 20% of the tumor cells display immunoexpression in the cytoplasm.27 The immunoexpression profiles of protein markers were evaluated in each case. In cases displaying dissimilarities between appendix and extra-appendiceal organ lesions, changes in protein marker patterns within any individual organs were regarded as alterations in the case.
Statistical Analysis
Pearson's χ2 and Fisher's exact tests were used to examine differences between the variables. Overall survival was measured from the date of diagnosis to death or last follow-up visit. Disease-free survival was calculated from the date of surgical resection to initial evidence of treatment failure. Patient survival rates were obtained using the Kaplan–Meier method, and differences in survival compared using the log-rank test. Multivariate analysis was performed using the Cox proportional hazards model. P-values of <0.05 were considered statistically significant. All statistical analyses were carried out using SPSS/PC version 12.0 for Windows (SPSS Inc., Chicago, IL, USA).
Results
Classification of Appendiceal Mucinous Neoplasms
The clinicopathological features of 70 appendiceal mucinous neoplasm cases are listed in Table 2. The 70 resected tumors were categorized using a three-tiered system, specifically, 32 mucinous adenomas, 23 mucinous neoplasms of uncertain malignant potential, and 15 mucinous adenocarcinomas (Table 2). In terms of mucinous neoplasm of uncertain malignant potential, we followed the definition of Pai et al4 (Figure 1). We adopted the mucinous adenocarcinoma definition of Carr et al2, 9 which included tumors displaying destructive invasion of the appendiceal wall, architectural complexity, cytologically high-grade atypia, or plump amount of epithelium outside the appendix (Figures 1 and 2). Interestingly, the proportions of tumor categories were strikingly different between tertiary-care and secondary-care hospitals. In the tertiary-care hospital (Seoul National University Hospital), 24, 42, and 34% mucinous adenoma, mucinous neoplasm of uncertain malignant potential, and mucinous adenocarcinoma cases were reported, respectively. Meanwhile, in two secondary-care hospitals, the proportions of mucinous adenoma, mucinous neoplasm of uncertain malignant potential, and mucinous adenocarcinoma were 76, 16, and 8% (Seoul National University Boramae Hospital) and 82, 12, and 6% (Bundang Seoul National University Hospital), respectively.
Differential Immunoexpression Profiles of Proteins in Mucinous Adenoma, Mucinous Neoplasm of Uncertain Malignant Potential and Mucinous Adenocarcinoma
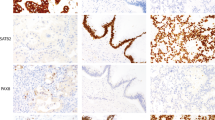
Among the 24 protein markers, 9 were more frequently altered in mucinous adenocarcinoma, compared to the mucinous adenoma group (Figure 3). Immunoexpression profiles of nine proteins (β-catenin, Cyclin Di, Ki-67, NF-κB, VEGF, E-cadherin, p53, MUC2, and MUC 5AC) according to tumor category are presented in Figure 4 and Table 3.
Representative immunohistochemical features of nine protein markers, which are more frequently altered in mucinous adenocarcinoma than mucinous adenoma (P<0.05). (a) Cyclin D1, nuclear positive. (b) Ki-67 (high labeling of nuclear positive). (c) NF-κB, nuclear positive. (d) VEGF, cytoplasmic positive. (e) E-cadherin, cytoplasmic membranous loss. (f) p53 overexpression (nuclear accumulation). (g) MUC2, cytoplasmic positive. (h) MUC5AC, cytoplasmic positive.
Differential immunoexpression patterns of proteins in appendiceal mucinous neoplasms according to tumor category. Immunoexpression of nine protein markers in mucinous adenoma, mucinous neoplasm of uncertain malignant potential, and mucinous adenocarcinoma groups, reveals significant differences (P<0.05). BENIGN, mucinous adenoma; UMP, mucinous neoplasm of uncertain malignant potential; MALIGNANT, mucinous adenocarcinoma.
The alteration frequencies of β-catenin, CyclinD1, Ki-67, E-cadherin, MUC2, and MUC5AC in mucinous neoplasm of uncertain malignant potential approached those in mucinous adenoma, whereas frequencies of NF-κB, VEGF and p53 were comparable to those in mucinous adenocarcinoma.
Discriminative Numbers of Altered Protein Markers in Mucinous Adenoma, Mucinous Neoplasms of Uncertain Malignant Potential, and Mucinous Adenocarcinoma Groups
The number of altered protein markers per case was determined. Mean numbers were significantly different among the three groups. Mucinous neoplasm of uncertain malignant potential was placed in the middle spectrum between mucinous adenoma and mucinous adenocarcinoma, with respect to the mean number of altered protein markers, estimated as 1.4 (median 1.0, range 0–5) in mucinous adenoma, 2.6 (median 2.0, range 0–6) in mucinous neoplasms of uncertain malignant potential, and 5.5 (median 5.0, range 3–8) in mucinous adenocarcinoma (P<0.05; Figure 5).
Number of altered protein markers among nine proteins in appendiceal mucinous neoplasms. Mean numbers of altered markers in mucinous adenoma, mucinous neoplasm of uncertain malignant potential and mucinous adenocarcinoma groups were 1.4, 2.6, and 5.5, respectively (P<0.05). BENIGN, mucinous adenoma; UMP, mucinous neoplasm of uncertain malignant potential; MALIGNANT, mucinous adenocarcinoma.
There were several cases, which unexpectedly showed marker alteration (Figure 5; Supplementary Information-1). A mucinous adenoma case (No. 27) displayed alterations in five markers. In this case, small mucinous adenoma (0.8 cm in tumor size) in appendix was observed incidentally, in the specimen undergone right hemicolectomy due to synchronous adenocarcinomas of the tubular type in ascending colon and cecum. In the mucinous neoplasm of uncertain malignant potential group, cases 34 and 38 displayed five altered marker proteins, and six altered markers were detected in case 44. Additionally, cases 37, 45, and 52 showed one altered marker, whereas case 47 presented no marker alterations. However, these cases displayed no specific features that could allow differentiation from other mucinous neoplasms of uncertain malignant potential patients, except that case 37 displayed mucinous adenoma-mimicking microscopic features, but a positive proximal resection margin.
Immunoexpression Status in the Appendix and Extra-appendiceal Organ in Mucinous Adenocarcinoma
In this study, extra-apendiceal organ involvement was pathologically confirmed in 14 out of 15 mucinous adenocarcinoma cases as follows: 8 cases in peritoneum, 8 in ovary, 7 in omentum, 3 in other intestinal segments, 3 in uterus, 2 in salpinx, 2 in spleen capsule, and 2 in regional lymph nodes.
In 10 cases, alterations in protein markers in the appendix and extra-appendiceal organ were slightly dissimilar. Moreover, the immunoexpression status within the mucinous adenocarcinoma group was not related to cytologic atypia, architecture complexity, or extent of extra-appendiceal organ involvement. For instance, case 68 displayed mucinous neoplasm of uncertain malignant potential-mimicking cytoarchitecture in the appendix per se, and more complex architecture in the ovary. However, this patient presented six altered markers in the appendix and four in the ovary. In two mucinous adenocarcinoma cases with high cytologic atypia, one displayed five and six altered markers in the appendix and extra-appendiceal lesion, whereas five altered markers each in the appendix and extra-appendiceal lesion were observed in the other case.
Clinical Significance of Immunoexpression Patterns in Mucinous Adenocarcinoma
In the mucinous adenocarcinoma group, the number of altered protein markers had an impact on clinical response. The mean number of altered protein markers in the mucinous adenocarcinoma group was 5.5, and median number, 5. The median number was rated as a cutoff, and clinical significance of the number of altered protein markers was evaluated. Eleven mucinous adenocarcinoma cases displayed alteration of five and more proteins. Treatment failure was reported for seven (64%) of these cases. Treatment failure was defined as metastasis to distant organs, recurrence, poor response to treatment or tumor-associated death after initial treatment at any time during the follow-up period. Meanwhile, four cases displayed alteration of four and less proteins, none of whom exhibited treatment failure. In univariate analysis, 11 cases with five and more protein alteration demonstrated a shorter period of disease-free survival than the 4 cases with four and less protein alteration, although the statistical significance was marginal (P=0.087; Figure 6a).
Disease-free survival plots in mucinous adenocarcinoma patients according to (a) number of altered protein markers; five and more altered markers (solid line) vs four and less altered markers (dotted line; P=0.087); (b) p53 status; overexpression (solid line) vs negative (dotted line; P=0.011); and (c) NF-κB status; nuclear positive (solid line) vs negative (dotted line; P=0.083).
In terms of clinical implications of individual markers, p53 alterations were associated with treatment failure in mucinous adenocarcinoma (P=0.041). Of the 15 mucinous adenocarcinoma cases, 6 displayed p53 overexpression, and treatment failure was reported for 5 (83%) of these cases. Meanwhile, only two (22%) of the nine p53-negative mucinous adenocarcinoma cases resulted in treatment failure. In univariate analysis, p53-overexpressing mucinous adenocarcinoma cases (n=6) showed a shorter period of disease-free survival than p53-negative cases (n=9; P=0.011; Figure 6b). In addition, NF-κB-positive mucinous adenocarcinoma cases (n=9) displayed a shorter period of disease-free survival than NF-κB- negative cases (n=6; P=0.083; Figure 6c). Regarding overall survival of patients, p53 overexpression was related to lower rate of overall survival (P=0.040), and β-catenin loss tended to show lower rate of overall survival (P=0.080; Figures 7a and b). Multivariate analysis disclosed no independent prognostic factors among protein markers.
Treatment failure was not reported for any of the mucinous adenoma and mucinous neoplasm of uncertain malignant potential cases during the follow-up period.
Discussion
We suggest that immunoexpression profiles of the nine protein markers are representative for appendiceal mucinous adenocarcinoma. Although appendiceal mucinous neoplasm accompanying peritoneal lesions are cytoarchitecturally benign in appearance, the molecular status of the tumor is consistent with features of mucinous adenocarcinoma, which are significantly different from those of mucinous adenoma or mucinous neoplasms of uncertain malignant potential. Meanwhile, once cases are classified as mucinous adenocarcinoma, the number of altered markers or individual marker alterations are not related to the cytoarchitecture grade of appendiceal and extra-appendiceal lesions, or extent of extra-appendiceal involvement. To date, few molecular studies on appendiceal mucinous neoplasms and even more limited immunohistochemistry analyses have been performed. To the best of our knowledge, limited information is available on mucin, E-cadherin, and p53 immunoexpression patterns focusing on both benign and malignant tumors.26
We propose that the number of altered protein markers, p53 status, NF-κB status, and β-catenin status are of clinical significance in appendiceal mucinous adenocarcinomas. In other words, p53 overexpression may signify shorter periods of disease-free survival and overall survival of patients. In addition, five and more altered markers and NF-κB positivity or β-catenin loss may be a sign of shorter periods of disease-free survival or overall survival, respectively.
In this study, a three-tiered classification of appendiceal mucinous neoplasms is supported by an analysis based on altered protein markers and clinical outcome. We adopted the three-tiered system suggested by Carr et al,2 and applied the strict guidelines of Carr et al2 and Pai et al4 to define mucinous neoplasms of uncertain malignant potential. In fact, Pai et al4 suggested a four-tiered system, specifically, mucinous adenoma, mucinous neoplasm of uncertain malignant potential, low malignant potential, and mucinous adenocarcinoma. Pai et al4 proposed the term low malignant potential for tumors that were cytoarchitecturally similar to mucinous adenoma, but neoplastic cells penetrated appendiceal wall and were present in peritoneal implants, and there was no metastasis. Taking into account this four-tiered system, 6 out of 15 mucinous adenocarcinoma cases in our study may be defined as low malignant potential. However, we cannot categorically discriminate between six cases of so-called low malignant potential and nine cases of higher grade atypia-mucinous adenocarcinoma, in terms of altered protein markers and clinical outcome (Supplementary Information-1 and -2). The above-described six cases may belong to low-grade mucinous neoplasm in two-tiered categorization system (low-grade mucinous neoplasm and mucinous adenocarcinoma) suggested by Misdraji et al.3 Like the preceding, so-called low-grade mucinous neoplasm and higher grade atypia-mucinous adenocarcinoma were not separated, based on altered protein markers and clinical outcome (Supplementary Information-1 and -2). The tumors of low-grade cytologic atypia (nucleomegaly, nuclear stratification, rare mitotic figures, single cell necrosis) and minimal architectural complexity (viliform, flat epithelial proliferation, small papillary excrescence) was designated as low-grade mucinous neoplasm by Misdraji et al,3 thus the concept of low-grade mucinous neoplasm encompasses mucinous adenoma through to low-grade atypia-mucinous adenocarcinoma. In daily practice, the term ‘low-grade mucinous neoplasm’ may be employed in ambiguous cases. However, we preferred a three-tiered categorization, along with a descriptive explanation, for instance, mucinous adenocarcinoma with high-grade or low-grade atypia.
In this study, 21 patients classified as mucinous neoplasm of uncertain malignant potential appeared close to mucinous adenoma, because no adverse clinical events were observed such as recurrence, metastasis, or high-grade transformation after initial treatment. In the series of Carr et al,2 2 out of 18 patients with mucinous neoplasm of uncertain malignant potential displayed evidence of recurrence, one at 6 months of follow-up and the other at 52 months after appendectomy. However, the first patient had a coexisting ‘ovarian mucinous tumor with low malignant potential’, which may have been an appendiceal mucinous adenocarcinoma involving ovary rather than a double organ primary tumor. In the study by Pai et al,4 late recurrences occurred in several patients with mucinous neoplasm of uncertain malignant potential during long-term follow-up. A small percentage of mucinous neoplasm of uncertain malignant potential tumors can develop into late recurrence. In our analysis, mucinous neoplasm of uncertain malignant potential cases exhibited differential protein immunoexpression profiles, which were distinguishable from those of benign and malignant appendiceal mucinous neoplasms. Hence, mucinous neoplasm of uncertain malignant potential is justified as a separate diagnostic entity in terms of clinical behavior and molecular changes.
Extra-appendiceal involvement (including pseudomyxoma peritonei) was additionally noted in 14 (18%) out of 70 patients, but only 3 (7%) out of 42 patients from secondary-care hospitals among our data. According to a 10-year nationwide study from the Netherlands, 9% of patients with appendiceal lesions developed pseudomyxoma peritonei.30 The proportion according to tumor category in a tertiary-care hospital was comparable to that in an AFIP series by Carr et al,2 ie. mucinous adenoma/mucinous neoplasm of uncertain malignant potential/mucinous adenocarcinoma was 24/42/34% in our data, and 28/17/55% in AFIP series.
In conclusion, immunoexpression profiling may be an effective complementary strategy in the classification of appendiceal mucinous neoplasms. Appendiceal mucinous adenoma, mucinous neoplasm of uncertain malignant potential, and mucinous adenocarcinoma are distinct in terms of protein marker alterations. In appendiceal mucinous adenocarcinoma cases, five and more altered protein markers, p53 overexpression, NF-κB positivisty, and β-catenin loss are factors associated with unfavorable clinical events, such as treatment failure, shorter period of disease-free survival, and shorter period of overall survival.
References
Young RH, Gilks B, Scully RE . Mucinous tumors of the appendix associated with mucinous tumors of the ovary and pseudomyxoma peritonei. A clinicopathological analysis of 22 cases supporting an origin in the appendix. Am J Surg Pathol 1991;15:415–429.
Carr NJ, McCarthy WF, Sobin LH . Epithelial noncarcinoid tumors and tumor-like lesions of the appendix. A clinicopathologic study of 184 patients with multivariate analysis of prognostic factors. Cancer 1995;75:757–768.
Misdraji J, Yantiss RK, Graeme-Cook FM, et al. Appendiceal mucinous neoplasms. A clinicopathological analysis of 107 cases. Am J Surg Pathol 2003;27:1089–1103.
Pai RK, Longacre TA . Appendiceal mucinous tumors and pseudomyxoma peritonei. Histologic Features, diagnostic problems, and proposed classification. Adv Anat Pathol 2005;12:291–311.
Bradley RF, Cortina G, Geisinger KR . Pseudomyxoma peritonei: review of the controversy. Curr Diag Pathol 2007;13:410–416.
Carr NJ, Arends MJ, Deans GT, et al. Adenocarcinoma of the appendix. In: Hamilton SR, Aaltonen LA (eds). World Health Organization Classification of Tumours: Pathology and Genetics of Tumours of the Digestive System. IARC Press: Lyon, 2000, pp 94–98.
Riddel RH, Petras RE, Williams GT, et al. Tumors of the Intestines. Atlas of Tumor Pathology. Third series. Fascicle 32. AFIP: Washinton, DC, 2003, pp 214–247.
Day DW, Jass JR, Price AB, et al. Morson and Dawson's Gastrointestinal Pathology, 4th edn, Blackwell Publishing: Oxford, 2003, pp 420–429.
Carr NJ, Emory TS, Sobin LH . Epithelial neoplasms of the appendix. In: Odze RD, Goldblum JR, Crawford JM (eds). Surgical Pathology of the GI Tract, Liver, Biliary Tract and Pancreas. Saunders: Philadelphia, PA, 2004, pp 473–478.
Rosai J . Rosai and Ackerman's Surgical Pathology, 9th edn, Mosby: Edinburgh, NY, 2004, pp 757–765.
Jass JR . Appendix. In: Fletcher CDM (ed). Diagnostic Histopathology of Tumors, 3rd edn, Churchill Livingstone: London, 2007, pp 390–391.
Fenoglio-Preiser, Cecilia M . Gastrointestinal Pathology: An Atlas and Text, 3rd ed, Wolters Kluwer/Lippincott Williams and Wilkins: Philadelphia, 2008, pp 525–541.
Leopoldo S, Lorena B, Cinzia A, et al. Two subtypes of mucinous adenocarcinoma of the colorectum: clinicopathological and genetic features. Ann Surg Oncol 2008;15:1429–1439.
Tozawa E, Ajioka Y, Watanabe H, et al. Mucin expression, p53 overexpression, and peritumoral lymphocytic infiltration of advanced colorectal carcinoma with mucus component: is mucinous carcinoma a distinct histological entity? Pathol Res Pract 2007;203:567–574.
Arai T, Kasahara I, Sawabe M, et al. Microsatellite-unstable mucinous colorectal carcinoma occurring in the elderly: comparison with medullary type poorly differentiated adenocarcinoma. Pathol Int 2007;57:205–212.
King-Yin Lam A, Ong K, Ho YH . Colorectal mucinous adenocarcinoma: the clinicopathologic features and significance of p16 and p53 expression. Dis Colon Rectum 2006;49:1275–1283.
Denkert C, Koch I, von Keyserlingk N, et al. Expression of the ELAV-like protein HuR in human colon cancer: association with tumor stage and cyclooxygenase-2. Mod Pathol 2006;19:1261–1269.
Li D, Semba S, Wu M, et al. Molecular pathological subclassification of mucinous adenocarcinoma of the colorectum. Pathol Int 2005;55:766–774.
Schönleben F, Allendorf JD, Qiu W, et al. Mutational analyses of multiple oncogenic pathways in intraductal papillary mucinous neoplasms of the pancreas. Pancreas 2008;36:168–172.
Miyasaka Y, Nagai E, Yamaguchi H, et al. The role of the DNA damage checkpoint pathway in intraductal papillary mucinous neoplasms of the pancreas. Clin Cancer Res 2007;13 (15 Part 1):4371–4377.
Ferreira CR, Carvalho JP, Soares FA, et al. Mucinous ovarian tumors associated with pseudomyxoma peritonei of adenomucinosis type: immunohistochemical evidence that they are secondary tumors. Int J Gynecol Cancer 2008;18:59–65.
Yoshida A, Sarian LO, Andrade LA, et al. Cell proliferation activity unrelated to COX-2 expression in ovarian tumors. Int J Gynecol Cancer 2007;17:607–614.
Chauhan SC, Singh AP, Ruiz F, et al. Aberrant expression of MUC4 in ovarian carcinoma: diagnostic significance alone and in combination with MUC1 and MUC16 (CA125). Mod Pathol 2006;19:1386–1394.
Logan-Collins JM, Lowy AM, Robinson-Smith TM, et al. VEGF expression predicts survival in patients with peritoneal surface metastases from mucinous adenocarcinoma of the appendix and colon. Ann Surg Oncol 2008;15:738–744.
Andreopoulou E, Yee H, Warycha MA, et al. Mucinous cancer of the appendix: challenges in diagnosis and treatment. J Chemother 2007;19:451–454.
Yajima N, Wada R, Yamagishi S, et al. Immunohistochemical expressions of cytokeratins, mucin core proteins, p53, and neuroendocrine cell markers in epithelial neoplasm of appendix. Hum Pathol 2005;36:1217–1225.
Chang MS, Lee HS, Kim WH, et al. Cell-cycle regulators, bcl-2 and NF-kappaB in Epstein- Barr virus-positive gastric carcinomas. Int J Oncol 2005;27:1265–1272.
Lee HS, Chang MS, Kim WH, et al. Epstein-barr virus-positive gastric carcinoma has a distinct protein expression profile in comparison with Epstein-Barr virus-negative carcinoma. Clin Cancer Res 2004;10:1698–1705.
Tan PH, Bay BH, Yip G, et al. Immunohistochemical detection of Ki67 in breast cancer correlates with transcriptional regulation of genes related to apoptosis and cell death. Mod Pathol 2005;18:374–381.
Smeenk RM, van Velthuysen ML, Verwaal VJ, et al. Appendiceal neoplasms and pseudomyxoma peritonei: a population based study. Eur J Surg Oncol 2008;34:196–201.
Acknowledgements
We thank SuperBioChips Laboratories (Seoul, Korea) for technical assistance. This work was supported by the Seoul National University Boramae Hospital Grant.
Author information
Authors and Affiliations
Corresponding author
Additional information
Supplementary Information accompanies the paper on Modern Pathology website (http://www.nature.com/modpathol)
Supplementary information
Rights and permissions
About this article
Cite this article
Yoon, S., Kim, Bh., Lee, H. et al. Differential protein immunoexpression profiles in appendiceal mucinous neoplasms: a special reference to classification and predictive factors. Mod Pathol 22, 1102–1112 (2009). https://doi.org/10.1038/modpathol.2009.74
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/modpathol.2009.74
Keywords
This article is cited by
-
Molecular and clinicopathological features of appendiceal mucinous neoplasms
Virchows Archiv (2021)