Abstract
The clinical significance of micropapillary growth pattern in ductal carcinoma in situ is controversial and the impact of nuclear grading in terms of recurrence of this lesion is yet to be clarified. Our aim was to evaluate, on a series of micropapillary in situ carcinomas, the histological features correlated with recurrence and whether the micropapillary subtype had a different behavior from other non-micropapillary ductal carcinoma in situ. We collected 55 cases of micropapillary in situ carcinomas from four institutions. All cases were reviewed for nuclear grade, extent, necrosis, microinvasion and tested for estrogen and progesterone receptors, Ki67, HER2, EGFR and p53 expression. Clinical data, type of surgery and follow up were obtained for all patients. Our results showed that the nuclear grade is crucial in determining the biology of micropapillary carcinoma in situ, so that the high nuclear grade micropapillary ductal carcinoma in situ more frequently overexpressed HER2, showed higher proliferation index, displayed necrosis and microinvasion and was more extensive than low/intermediate nuclear grade. Logistic regression analysis confirmed the high nuclear grade (Odds ratio: 6.86; CI: 1.40–33.57) as the only parameter associated with elevated risk of local recurrence after breast-conserving surgery. However, the recurrence rate of 19 micropapillary carcinoma in situ, which were part of a cohort of 338 consecutive ductal carcinoma in situ, was significantly higher (log-rank test, P-value=0.019) than that of non-micropapillary, independently of the nuclear grade. In conclusion, although nuclear grade may significantly influence the biological behavior of micropapillary ductal carcinoma in situ, micropapillary growth pattern per se represents a risk factor for local recurrence after breast-conserving surgery.
Similar content being viewed by others
Main
In recent years, ductal carcinoma in situ of the breast has been increasingly diagnosed because of the widespread use of mammography in asymptomatic women, and it now accounts for about 20% of all breast cancers in screened populations.1, 2, 3 The proposed standard treatment for ductal carcinoma in situ has radically changed, moving from radical mastectomy towards breast-conserving surgery, with either excision alone or excision followed by radiotherapy.4 However, long-term follow-up analyses indicate that approximately 10–15% of women affected by ductal carcinoma in situ and treated with conserving surgery plus radiation therapy may develop local recurrences5, 6 of either in situ or invasive carcinoma, with an increased risk of distant metastasis and death because of the disease.7, 8, 9
Thus, several studies have been performed to identify those patients harboring ductal carcinoma in situ that is likely to recur or progress to invasive carcinoma and should therefore be treated by radical mastectomy.5, 6, 7, 8, 9, 10, 11, 12, 13, 14, 15, 16, 17, 18, 19 Some clinico-pathological data of ductal carcinoma in situ such as age of the patient, size of the lesion, method of detection, comedo-necrosis, multifocality, margin status and nuclear grade have been evidenced to be strong predictors of recurrences.12, 14, 15, 16, 17, 19 Conversely, the clinical relevance of histological growth patterns is still debated. Commonly, the ‘cribriform’ growth pattern is related to indolent lesions with a low risk of subsequent invasive carcinoma, whereas solid and comedo types of ductal carcinoma in situ are known as aggressive lesions.5, 7, 18 The biological potential of micropapillary in situ carcinoma is still questioned. Scott et al20 were the first to propose to classify micropapillary in situ cancer as a ‘special type’, excluding it from the low-, intermediate- and high-grade categories of ductal carcinoma in situ because of its likelihood of extensive disease. Mack et al21 confirmed that micropapillary in situ carcinoma tends to involve the ducts extensively when present in pure form, regardless of the nuclear grade. Other authors have shown that the micropapillary type is significantly associated with multicentricity and microinvasion when compared with other types.22 In addition, a recent study showed micropapillary growth pattern to be an independent high risk factor for local recurrences.11 On the other hand, a previous study5 assimilated well-differentiated low-grade micropapillary ductal carcinoma in situ to ‘clinging’ carcinomas and suggested that excision without additional irradiation may be offered to the patients, considering the exceptionally low risk of recurrences of these in situ cancers; whereas other studies on the role of sentinel nodes in ductal carcinoma in situ have shown that micrometastases were regularly associated to non-high grade micropapillary in situ variant.23, 24, 25, 26
Taking all these data together, we collected a series of micropapillary ductal carcinoma in situ with the aim to evaluate the biological and clinical significance of this growth pattern.
Materials and methods
We retrieved 55 ductal carcinoma in situ showing a growth pattern of epithelial micropapillae in more than 95% of the ducts from the pathology files of the San Giovanni Battista-Molinette Hospital and S Anna Hospital in Turin, AOU Careggi Hospital in Florence, and the Central Hospital in Falun. Patients’ clinical data including age, presence of nipple discharge, presence of Paget's disease, treatment (type of surgery and radiotherapy) were collected for each case. Follow-up information was censored at the time of death or loss to follow-up. All cases were centrally reviewed by two pathologists (AS and IC). Nuclear grade was reviewed according to standard nuclear grading criteria.27 Details on the presence of necrosis and microinvasion were assessed during the review as well. Microinvasion was defined as one focus of invasive carcinoma <1 mm in diameter, visible on no more than two consecutive sections of the same block. Three cases with larger foci of invasion or multiple foci of microinvasion were excluded from the study ab initio. The extent of disease and the nearest margin width were recorded in millimeters, using the data of the original report. Only the Falun Institute routinely performed large section processing.28 As a common rule, in the other institutions, the surgical specimen obtained from a large excision was completely examined at histology following a mapping procedure that allowed a reconstruction of the tumor extent. Multiple sampling of all calcifications, visible on radiograms of surgical samples, were performed for specimens larger than 10 cm or for mastectomy specimens. The modified Van Nuys Prognostic Index was calculated as a product of a patient's age, tumor extent, margin width and nuclear grade, as previously described.29
To analyze the effect of the micropapillary growth pattern on clinical outcome, we compared micropapillary to non-micropapillary ductal carcinoma in situ. For this aim, we focused on 19 of the 55 micropapillary carcinomas, identified within a cohort of 338 (6%) consecutive ductal carcinoma in situ, referred by the population regional mammography screening program and post-operatively diagnosed at San Giovanni Battista-Molinette Hospital and Sant’Anna Hospital in Turin between 1992 and 2005. Patients had no previous (as assessed by link with the Piedmont Cancer Registry files) or concomitant invasive cancer. Slides were reviewed by one of the authors (IC) who was blind to the original diagnosis and the recurrence status. Eligible cases were originally 422, 69 of which were excluded for unavailability of the histopathology block and 15 being re-classified as invasive cancer (invasion >1 mm), or atypical ductal, or lobular hyperplasia.27
Nuclear grade, presence of necrosis and microinvasion were assessed at revision and recorded for all cases. Tumor extension and margin status as well as patients’ clinical data such as age and treatment (type of surgery and radiotherapy) were obtained from the clinical records and from regional ambulatory care files. Follow-up information on life status (Civil Status files) and disease status (follow-up clinics, regional hospital discharge and regional ambulatory care files) was collected for each case. Follow-up was updated to February 2008 and was censored at the time of death or loss to follow-up.
Immunohistochemistry
Sections of the representative tumor blocks were cut at 4 μm and mounted on silane-coated slides. Immunohistochemistry was performed using an automated immunostainer (Ventana BenchMark AutoStainer, Ventana Medical Systems, Tucson, AZ, USA) with antibodies against estrogen receptor (SP1 rabbit monoclonal, prediluted Ventana), progesterone receptor (1E2 monoclonal, prediluted Ventana), Ki67 (1:100 diluted, Mib1, DAKO), HER2 (1:800 diluted, DAKO, Glostrup, Denmark), p53 (DO-7 prediluted Ventana) and epidermal growth factor receptor (1:50 diluted, 31G7 Zymed). HER2 expression was scored on a scale from 0 to 3 according to the recommended guidelines for invasive carcinoma.30 Fluorescence in situ hybridization (FISH) was performed on equivocal cases scored as 2+ by immunohistochemistry. Cases were considered HER2 positive if they had a 3+ score or showed HER2 amplification. Positive and negative controls were included for each immunohistochemical run.
Fluorescence In Situ Hybridization
HER2 gene analysis was carried out by FISH using the PathVysion HER2/neu probe kit (Vysis, Downers Grove, IL, USA) as previously described.31 Briefly, sections were incubated overnight at 56°C, dewaxed in xylene, dehydrated in 100% ethanol and air-dried. Slides were then treated with proteases for 45–60 min, denatured at 97°C, and hybridized overnight at 37°C. Slides were washed with post-hybridization buffer at 72°C, counterstained with 40,60-diamidino-2-phenylindole (DAPI), mounted, and stored in the dark before counting. Automated acquisition was performed with the motorized Metafer Scanning System (Carl Zeiss MetaSystems GmbH) and AxioImager epifluorescent microscope (1 focus plane for DAPI and 13 focus planes for green and red spots), and FISH evaluation was performed using the Metafer through the PathVysion V2 software (FDA approved). Amplification was defined as a final HER2/CEP17 ratio >2.2.
Statistical Analysis
The Pearson's χ2-test was employed to compare proportions, except when the Fisher's exact test was appropriate because of small sample size. Student's t-test was used for continuous variables (extent, age) and the Mann–Whitney U-test for non-parametric variables (median). The log-rank test was used to assess differences between groups when comparing the time to recurrence.
Time to first ipsilateral recurrence (ductal carcinoma in situ or invasive carcinoma) was analyzed using Kaplan–Meier curves. To adjust the risk estimate for potential confounders, a Cox proportional hazard model was used and hazard ratios were calculated. Follow-up was censored at the time of death or the last clinical investigation of the patient. Statistical analysis was carried out using the SPSS version 13.0 (SPSS Chicago, IL, USA) software and the R environment (www.r-project.org).
Results
Histopathological and Clinical Characteristics of 55 Micropapillary Ductal Carcinoma In Situ
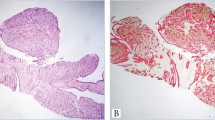
The mean age of patients at diagnosis was 57.2 years. All cases were diagnosed for radiological calcifications. Three patients presenting with nipple discharge had positive cytological smears (Figure 1). None had Paget's disease. The extent of the whole series of micropapillary ductal carcinoma in situ calculated as the mean dimension obtained from the pathology report was 30 mm. However, the extent of 10 cases measured on large-format histology slides at Falun Central Hospital was considerably larger (mean 68 mm).
Thirteen (24%), 16 (29%) and 26 (47%) cases were classified as low, intermediate and high nuclear grade in situ carcinoma, respectively. Low nuclear grade micropapillary tumor showed ducts lined by numerous club-like micropapillae formed by monomorphic cells with rounded, uniform and equidistant nuclei, slightly larger than in normal ductal epithelial cells. The chromatin was finely dispersed. The nucleoli were inconspicuous and the mitotic figures were absent. (Figure 2a and b). None of the patients with low nuclear grade in situ cancer had nipple discharge. In the intermediate nuclear grade micropapillary group, moderately atypical cells, displaying pleomorphic nuclei with increased nuclear/cytoplasmic ratio, occasional nucleoli and coarse chromatin, were arranged in micropapillae of a different shape (Figure 2c and d). High nuclear grade micropapillary in situ carcinomas showed irregular micropapillae formed by atypical cells with large, markedly pleomorphic, poorly polarized nuclei and apparent nucleoli. Mitotic figures and necrotic cellular debris in the duct lumen were common features (Figure 2e and f). Some histological calcifications were present in all cases; however large amorphous calcifications prevailed in high nuclear grade lesions. Nipple discharge was observed in one patient with intermediate and in two patients with high nuclear grade micropapillary tumors.
Representative micrographs of micropapillary ductal carcinoma in situ. Low nuclear grade micropapillary ductal carcinoma in situ: numerous club-like micropapillae are formed by monomorphic cells with rounded, uniform, and equidistant nuclei. The chromatin is finely dispersed. Mitotic figures are absent (a and b). Intermediate nuclear grade micropapillary ductal carcinoma in situ: micropapillae of different shapes are formed by cells displaying pleomorphic nuclei with increased nuclear/cytoplasmic ratio, occasional nucleoli, and coarse chromatin (c and d). High nuclear grade micropapillary ductal carcinoma in situ: irregular micropapillae are formed by atypical cells with large, markedly pleomorphic, poorly polarized nuclei and apparent nucleoli. Mitotic figures and necrotic cellular debris are present (e and f). Micropapillary structures are seen floating within the lumen of ducts regardless of the nuclear grade.
Age, extent, necrosis and microinvasion, according to the nuclear grade of micropapillary in situ lesions, are shown in Table 1. High-grade tumors were significantly larger and, more frequently than non-high grade lesions, showed necrosis and microinvasion.
As expected, the expression of estrogen receptor and progesterone receptor was significantly higher in low and intermediate grade carcinomas, whereas HER2 and Ki67 expression prevailed in high-grade micropapillary lesions. The HER2 gene was amplified in FISH analysis in three of the six cases that were scored as 2+ in immunohistochemistry. p53 and epidermal growth factor receptor overexpression was prevalent in high-grade micropapillary as well; however, this difference was not statistically significant (Table 1).
The mean follow up of the whole series was 74.3 months. Sentinel node surgery was not performed in any of the patients. None of the patients received adjuvant endocrine therapy.
Nineteen of 55 (34%) patients underwent mastectomy and two of these suffered from nipple discharge. The only parameter associated with the decision to perform mastectomy was the extent of the lesion (Table 2).
Of the 36 patients treated with conserving surgery, 23 of them (64%) received radiotherapy. Ipsilateral recurrence occurred in 11 patients (31%), 7 of whom had invasive carcinoma (mean follow-up time 74.3 months). The mean time to recurrence was 47.67 months (median 50). Four out of four cases presenting a surgical-free margin <1 mm in width did not recurr, whereas five cases with a surgical-free margin >10 mm recurred after conserving surgery. Radiation therapy did not influence the recurrence rate. In univariate analysis only high nuclear grade significantly correlated with recurrence (Table 3).
Comparison between Micropapillary and Non-Micropapillary Ductal Carcinoma In Situ
Mean age was comparable in the two groups: 58.5 years in patients with micropapillary and 59.6 in patients with non-micropapillary ductal in situ, carcinoma. The percentage of cases with high nuclear grade was significantly higher in micropapillary tumors. They also showed greater extent but this difference was not statistically significant, probably because of some underestimation due to traditional sampling procedures (Table 4).
The mastectomy rate was similar in patients with micropapillary (21%) and non-micropapillary carcinomas (14%). Among the 288 women treated with conserving surgery, the only difference between the two groups was the nuclear grade, that was higher in micropapillary (64%) in comparison with non-micropapillary ductal carcinoma in situ (35%). No other significant differences were found (Table 5). The median follow-up time of patients treated by breast-conserving surgery was 69.13 months (range 13.80–170.28 months) in the micropapillary group and 71.20 months (range 4.83–178.50 months) in the non-micropapillary in situ cancers (P-value=0.838 according to the Mann–Whitney U-test). One patient died of breast cancer in the non-micropapillary group, none within the micropapillary cases.
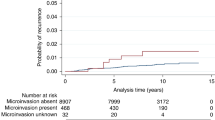
Ipsilateral recurrence occurred in 4 of 14 (29%) patients with micropapillary (two cases recurring with micropapillary in situ carcinomas and two cases developing invasive carcinoma) vs 23 of 274 (8%) of patients with non-micropapillary cancers (Fisher's exact test P-value=0.032). Three of the recurring cases of micropapillary carcinomas were of high nuclear grade, with an extent ranging from 10 to 42 mm and free margin >10 mm. Two received adjuvant radiotherapy. One lesion of intermediate nuclear grade was devoid of necrosis with an extent of 12 mm and a margin width <1 mm and did not receive radiation therapy. A significantly higher recurrence rate for micropapillary in situ carcinomas was found using the Kaplan–Meyer analysis (log-rank test, P-value=0.019) (Figure 3). In univariate analysis, none of the variables in Table 5 were significantly associated with recurrence, with the lowest P-values achieved by nuclear grade (0.114) and radiotherapy (0.149). However, nuclear grade and radiation therapy were included as covariates in the multivariate model of the Cox proportional hazard analysis, because they were identified as potential confounders a priori from literature data and because they showed the greatest association and the lowest P-values in relation to exposure (micropapillary status) and/or outcome (ipsilateral recurrence). The crude and adjusted hazard ratio were 3.32 (P-value=0.027) and 3.01 (P-value=0.046), respectively, thus confirming, in our series, a higher risk of recurrence for micropapillary histotype.
Discussion
In this study, we showed that micropapillary as a sole growth pattern is rarely encountered (6%) within ductal carcinoma in situ,20, 21, 22, 32 however, we here proved that this histotype merits specific surgical attention because of the high risk of recurrence.
Micropapillary in situ carcinoma has been considered as a special type of ductal carcinoma in situ independently from nuclear grade.20 We showed that nuclear grade influences the biological features (extent, necrosis, microinvasion and immunophenotype) of micropapillary carcinoma in situ. In the text book by Rosen,33 micropapillary in situ neoplasms are classified as well and poorly differentiated, the latter including tumors of intermediate and high cytomorphological grade. In our study, the immunophenotypical analysis confirmed that an intermediate nuclear grade of micropapillary in situ carcinomas does exist; however, from the clinical point of view, focusing on recurrence rate, it can be assimilated to the low rather than into the high nuclear grade. Previous authors34 showed that about 50% of micropapillary in situ carcinomas, not further classified, overexpressed HER2 and that HER2 was phosphorylated in 30% of these cases. Our results showed that 77% of high nuclear grade micropapillary in situ tumors overexpressed HER2. In addition, high nuclear grade tumors were significantly more extensive and more frequently related to ipsilateral recurrence after conserving surgery than low and intermediate nuclear grade micropapillary type. In addition, all cases associated with microinvasion were of high nuclear grade. Microinvasion could be considered as a distraction in this study, but as shown by other authors foci of invasion <1 mm have low or no impact on recurrence in ductal carcinomas in situ as compared with inadequate local control.35, 36, 37
On the other hand, the recurrence rate of micropapillary in situ lesions per se was higher than that of non-micropapillary in situ neoplasms, independently of the nuclear grade, microinvasion and any other parameter including the margin status and the adjuvant radiation therapy. A possible explanation for this clinical behavior may be that micropapillae may detach, roll within the ducts, and colonize far away from the site of origin, giving rise to recurrence (Figure 3). This peculiar intraductal dissemination of micropapillae would also justify the difficulties in defining the surgical margin status, as tracts of ducts may be skipped between two foci of micropapillary in situ carcinomas and therefore lead to a false interpretation of free margins on surgical specimens. In fact, in the present series, the margin status did not show correlation with ipsilateral recurrence. Finally, the hypothesis of intraductal dissemination of the micropapillae would also give reason to the presence of neoplastic cells in nipple secretion38 and to the rare occurrence of Paget disease.39 For example three patients with micropapillary in situ carcinoma in the present series suffered from nipple discharge and the cytological smears contained neoplastic cells. Another possibility for the wide spreading of this lesion is that micropapillary structures may develop simultaneously on a large area of a breast lobe.40 The extensive nature of the micropapillary in situ cancer was first reported by Patchefsky et al22 and confirmed by other authors.20, 21 Bellamy et al32 described that micropapillary growth, more likely than other growth patterns of in situ carcinoma, involves multiple quadrants, regardless of nuclear grade or necrosis. In addition, previous studies reported that micropapillary in situ cancer may be completely silent on mammograms or may produce typical snake skin-like microcalcifications, causing frequent discrepancies in radiological vs pathological assessment,41 and that they may be totally silent on magnetic resonance imaging as well, even if they are extensive and of high-grade.42 These peculiar radiological features should be considered when sampling for histopathological examination is performed on the basis of calcifications, as shown on the radiogram of surgical specimen. Standard sampling may in effect underestimate the wide spreading of micropapillary in situ carcinomas and the involvement of surgical margins as shown in this series. The definitely larger mean extent of micropapillary in situ carcinomas reported in the single institution performing large-format histology slides confirms the limit in measuring the real dimension of micropapillary carcinoma in situ.
In conclusion, our results confirmed that micropapillary ductal carcinoma in situ represents a biologically remarkable subset of in situ carcinoma and that large excision should be considered independently from the nuclear grade and the extent of radiological calcifications when this growth pattern is diagnosed.
References
Ernster VL, Ballard-Barbash R, Barlow WE, et al. Detection of ductal carcinoma in situ in women undergoing screening mammography. J Natl Cancer Inst 2002;94:1546–1554.
Leonard GD, Swain SM . Ductal carcinoma in situ, complexities and challenges. J Natl Cancer Inst 2004;96:906–920.
May DS, Lee NC, Nadel MR, et al. The National Breast and Cervical Cancer Early Detection Program: report on the first 4 years of mammography provided to medically underserved women. AJR Am J Roentgenol 1998;170:97–104.
Fisher B, Wickerham DL, Deutsch M, et al. Breast tumor recurrence following lumpectomy with and without breast irradiation: an overview of recent NSABP findings. Semin Surg Oncol 1992;8:153–160.
Bijker N, Meijnen P, Peterse JL, et al. Breast-conserving treatment with or without radiotherapy in ductal carcinoma-in-situ: ten-year results of European Organisation for Research and Treatment of Cancer randomized phase III trial 10853—a study by the EORTC Breast Cancer Cooperative Group and EORTC Radiotherapy Group. J Clin Oncol 2006;24:3381–3387.
Fisher B, Costantino J, Redmond C, et al. Lumpectomy compared with lumpectomy and radiation therapy for the treatment of intraductal breast cancer. N Engl J Med 1993;328:1581–1586.
Fisher ER, Dignam J, Tan-Chiu E, et al. Pathologic findings from the National Surgical Adjuvant Breast Project (NSABP) eight-year update of Protocol B-17: intraductal carcinoma. Cancer 1999;86:429–438.
Mokbel K, Cutuli B . Heterogeneity of ductal carcinoma in situ and its effects on management. Lancet Oncol 2006;7:756–765.
Warnberg F, Bergh J, Zack M, et al. Risk factors for subsequent invasive breast cancer and breast cancer death after ductal carcinoma in situ: a population-based case-control study in Sweden. Cancer Epidemiol Biomarkers Prev 2001;10:495–499.
Fisher B, Dignam J, Wolmark N, et al. Tamoxifen in treatment of intraductal breast cancer: National Surgical Adjuvant Breast and Bowel Project B-24 randomised controlled trial. Lancet 1999;353:1993–2000.
Fisher ER, Land SR, Saad RS, et al. Pathologic variables predictive of breast events in patients with ductal carcinoma in situ. Am J Clin Pathol 2007;128:86–91.
Lagios MD . Heterogeneity of duct carcinoma in situ (DCIS): relationship of grade and subtype analysis to local recurrence and risk of invasive transformation. Cancer Lett 1995;90:97–102.
Mechera R, Viehl CT, Oertli D . Factors predicting in-breast tumor recurrence after breast-conserving surgery. Breast Cancer Res Treat 2009;116:171–177.
Ottesen GL, Graversen HP, Blichert-Toft M, et al. Ductal carcinoma in situ of the female breast. Short-term results of a prospective nationwide study. The Danish Breast Cancer Cooperative Group. Am J Surg Pathol 1992;16:1183–1196.
Rakovitch E, Pignol JP, Hanna W, et al. Significance of multifocality in ductal carcinoma in situ: outcomes of women treated with breast-conserving therapy. J Clin Oncol 2007;25:5591–5596.
Silverstein MJ, Buchanan C . Ductal carcinoma in situ: USC/Van Nuys Prognostic Index and the impact of margin status. Breast 2003;12:457–471.
Silverstein MJ, Lagios MD, Craig PH, et al. A prognostic index for ductal carcinoma in situ of the breast. Cancer 1996;77:2267–2274.
Solin LJ, Fourquet A, Vicini FA, et al. Mammographically detected ductal carcinoma in situ of the breast treated with breast-conserving surgery and definitive breast irradiation: long-term outcome and prognostic significance of patient age and margin status. Int J Radiat Oncol Biol Phys 2001;50:991–1002.
Warnberg F, Nordgren H, Bergh J, et al. Ductal carcinoma in situ of the breast from a population-defined cohort: an evaluation of new histopathological classification systems. Eur J Cancer 1999;35:714–720.
Scott MA, Lagios MD, Axelsson K, et al. Ductal carcinoma in situ of the breast: reproducibility of histological subtype analysis. Hum Pathol 1997;28:967–973.
Mack L, Kerkvliet N, Doig G, et al. Relationship of a new histological categorization of ductal carcinoma in situ of the breast with size and the immunohistochemical expression of p53, c-erb B2, bcl-2, and ki-67. Hum Pathol 1997;28:974–979.
Patchefsky AS, Schwartz GF, Finkelstein SD, et al. Heterogeneity of intraductal carcinoma of the breast. Cancer 1989;63:731–741.
Cserni G . Sentinel lymph node biopsy as a tool for the staging of ductal carcinoma in situ in patients with breast carcinoma. Surg Today 2002;32:99–103.
Lara JF, Young SM, Velilla RE, et al. The relevance of occult axillary micrometastasis in ductal carcinoma in situ: a clinicopathologic study with long-term follow-up. Cancer 2003;98:2105–2113.
Pendas S, Dauway E, Giuliano R, et al. Sentinel node biopsy in ductal carcinoma in situ patients. Ann Surg Oncol 2000;7:15–20.
Zavagno G, Carcoforo P, Marconato R, et al. Role of axillary sentinel lymph node biopsy in patients with pure ductal carcinoma in situ of the breast. BMC Cancer 2005;5:28.
Tavassoli FA, Hoefler H, Rosai J, et al. Intraductal proliferative lesions In: Tavassoli FA, Devilee P (eds). Pathology and Genetics of Tumours of the Breast and Female Genital Organs Vol. IARC Press: Lyon, 2003.
Tot T, Tabar L . Mammographic-pathologic correlation of ductal carcinoma in situ of the breast using two- and three-dimensional large histologic sections. Semin Breast Dis 2006;8:144–151.
Silverstein MJ . The University of Southern California/Van Nuys prognostic index for ductal carcinoma in situ of the breast. Am J Surg 2003;186:337–343.
Wolff AC, Hammond ME, Schwartz JN, et al. American Society of Clinical Oncology/College of American Pathologists guideline recommendations for human epidermal growth factor receptor 2 testing in breast cancer. J Clin Oncol 2007;25:118–145.
Castellano I, Sapino A, Arisio R, et al. Fluorescent in situ hybridization as a screening test for HER2 amplification in G2 and G3 breast cancers of lobular and ductal histotype and metastases. Oncol Rep 2008;19:1271–1275.
Bellamy CO, McDonald C, Salter DM, et al. Noninvasive ductal carcinoma of the breast: the relevance of histologic categorization. Hum Pathol 1993;24:16–23.
Rosen P . Rosen's Breast Pathology. Lippincott Williams & Wilkins: Philadelphia, PA, 2009.
DiGiovanna MP, Chu P, Davison TL, et al. Active signaling by HER-2/neu in a subpopulation of HER-2/neu-overexpressing ductal carcinoma in situ: clinicopathological correlates. Cancer Res 2002;62:6667–6673.
Padmore RF, Fowble B, Hoffman J, et al. Microinvasive breast carcinoma: clinicopathologic analysis of a single institution experience. Cancer 2000;88:1403–1409.
Silver SA, Tavassoli FA . Mammary ductal carcinoma in situ with microinvasion. Cancer 1998;82:2382–2390.
Wong JH, Kopald KH, Morton DL . The impact of microinvasion on axillary node metastases and survival in patients with intraductal breast cancer. Arch Surg 1990;125:1298–1301; discussion 301–2.
Bauer RL, Eckhert Jr KH, Nemoto T . Ductal carcinoma in situ-associated nipple discharge: a clinical marker for locally extensive disease. Ann Surg Oncol 1998;5:452–455.
Vielh P, Validire P, Kheirallah S, et al. Paget's disease of the nipple without clinically and radiologically detectable breast tumor. Histochemical and immunohistochemical study of 44 cases. Pathol Res Pract 1993;189:150–155.
Tot T . DCIS, cytokeratins, and the theory of the sick lobe. Virchows Arch 2005;447:1–8.
Tabar L, Vitak B, Chen HH, et al. The Swedish Two-County Trial twenty years later. Updated mortality results and new insights from long-term follow-up. Radiol Clin North Am 2000;38:625–651.
Tot T, Gere M . Radiological-pathological correlation in diagnosing breast carcinoma: the role of pathology in the multimodality era. Pathol Oncol Res 2008;14:173–178.
Acknowledgements
This work was supported by PRIN 2005, Regione Piemonte CIPE 2004, Regione Piemonte Ricerca Sanitaria Finalizzata 2008 e 2008 bis, and AIRC 2007–2008 research fund grants. We are grateful to Compagnia San Paolo and Fondazione Cassa di Risparmio di Torino for funding supports. We acknowledge the following people: Dr Elda Mano for the follow-up of the population series; Dr Alessia Reali, Dr Sergio Gribaudo, and Dr Paolo Rovea for kindly researching and providing supplementary information on RT; and Mrs Aurora Di Leo for her contribution to data collection.
Author information
Authors and Affiliations
Corresponding author
Additional information
Disclosure/conflict of Interest
The authors declare no conflict of interest.
Rights and permissions
About this article
Cite this article
Castellano, I., Marchiò, C., Tomatis, M. et al. Micropapillary ductal carcinoma in situ of the breast: an inter-institutional study. Mod Pathol 23, 260–269 (2010). https://doi.org/10.1038/modpathol.2009.169
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/modpathol.2009.169
Keywords
This article is cited by
-
Primary tumor location predicts the site of local relapse after nipple–areola complex (NAC) sparing mastectomy
Breast Cancer Research and Treatment (2017)