Abstract
The expression of estrogen receptor-α (ER-α) and related genes has emerged as one of the major determinants of molecular classification of invasive breast cancers. Expression of a second ER, estrogen receptor-β (ER-β), has not been previously evaluated in a large population-based study. Therefore, we examined ER-β expression in a large population of women with breast cancer to assess its relationship to molecular categories of invasive breast cancer. We constructed tissue microarrays from paraffin blocks of 3093 breast cancers that developed in women enrolled in the Nurses' Health Study. Tissue microarray sections were immunostained for ER-α, progesterone receptor (PR), human epidermal growth factor receptor 2 (HER2), cytokeratin 5/6, epidermal growth factor receptor (EGFR) and with a monoclonal antibody to ER-β. Cancers were categorized as luminal A (ER-α+ and/or PR+ and HER2–); luminal B (ER-α+ and/or PR+ and HER2+); HER2 (ER-α− and PR− and HER2+); and basal-like (ER-α−, PR−, HER2− and EGFR or cytokeratin 5/6+). The relationship between expression of ER-β and molecular class of invasive breast cancer was analyzed. Overall, 68% of breast carcinomas were ER-β+. Expression of ER-β was significantly associated with expression of ER-α (P<0.0001) and PR (P<0.0001), and was inversely related to expression of HER2 (P=0.004), CK5/6 (P=0.02) and EGFR (P=0.006). Among 2170 invasive cancers with complete immunophenotypic data, 73% were luminal A, 5% luminal B, 6 % HER2 and 11% basal-like. ER-β expression was significantly related to molecular category (P<0.0001) and was more common in luminal A (72% of cases) and B (68% of cases) than in HER2 or basal-like types. However, despite their being defined by the absence of ER-α expression, 55% of HER2-type and 60% of basal-like cancers showed expression of ER-β. The role of ER-β in the development and progression of breast cancers defined by lack of expression of ER-α merits further investigation.
Similar content being viewed by others
Main
Recent studies using microarray technology and unsupervised cluster analysis have provided new insights into the classification of invasive breast cancers.1, 2, 3, 4 These studies have resulted in the identification of several breast cancer subgroups that vary in their gene expression signatures and clinical course. The molecularly distinct breast cancer subgroups that have been the most reproducibly identified to date include luminal subtypes A and B (both of which are hormone receptor positive), the human epidermal growth factor receptor 2 (HER2) subtype and a group known as basal-like cancers.1, 2, 3, 4 Using this approach, the expression of estrogen receptor-α (ER-α) and related genes has emerged as one of the major determinants in defining molecular category, and ER-α is the primary target determining whether patients should receive hormonal therapy.
A second estrogen receptor, estrogen receptor-β (ER-β), was discovered in 1996.5 Since then there has been increasing interest in its role in human breast cancers. Although its precise biologic role remains unclear, in part due to the fact that several isoforms exist, recent studies examining ER-β have suggested that its expression may be a prognostic factor and predictive factor in patients with breast cancer.6, 7, 8, 9, 10, 11, 12, 13 In addition, there is mounting evidence to support a role for ER-β in breast cancer onset and progression.6, 14, 15
Estrogen receptor-β is highly expressed in normal breast epithelium and its expression has been reported to decrease with tumor progression from in situ through to invasive carcinomas.16, 17 Nevertheless, up to 75% of invasive breast cancers have been shown to express ER-β, depending on the method used for its detection.11, 12, 14, 18, 19, 20, 21 Of note, a subset of tumors that are ER-α− are ER-β+, and this may have implications for new therapeutic options for these patients.10, 22, 23, 24
The expression of ER-β among the molecularly defined categories of invasive breast cancer has only been evaluated in smaller studies.10, 22, 23, 24 Therefore, using a large, well-characterized population of women with breast cancer, the objective of this study was to examine the expression of ER-β in relation to molecular phenotype.
Materials and methods
Study Population
Study design and population
The Nurses' Health Study was initiated in 1976, when 121 700 US registered nurses aged 30–55 returned an initial questionnaire. The cohort has been followed by mailed questionnaires biennially to update exposure information and ascertain nonfatal incident diseases. Information on body mass index, reproductive history, age at menopause and postmenopausal hormone use as well as diagnosis of cancer and other diseases are updated every 2 years through questionnaires. The follow-up rate among this cohort was over 90% through 1996.
Breast Cancer Case Confirmation
All women reporting incident diagnoses of cancer were asked for permission to review their medical records to confirm the diagnosis and to classify cancers as in situ or invasive, by histologic type, size and the presence or absence of metastases. To identify cases of cancer in nonrespondents who died, we obtained death certificates for all deceased participants and medical records for the incident cancers. Following medical record review, 99% of self-reported breast cancers were confirmed.
Breast Cancer Tissue Block Collection
In 1993, we began collecting archived formalin-fixed paraffin-embedded breast cancer blocks for participants with primary incident breast cancers over 20 years of follow-up (1976–1996). Cases who reported a prevalent cancer including breast cancer at baseline were excluded from collection. Of the 5610 breast cancers that were eligible for block collection, we were unable to obtain any pathology material for 1858 cases. The primary reason was because they had been destroyed by the hospital (45%). Because the majority of hospitals archive tissue blocks for only 5–10 years, we were more successful in obtaining more recent blocks. Because year of diagnosis and age at diagnosis are highly correlated (Spearman's correlation=0.49; P<0.0001), the temporal effect on our collections is evident not only in the differences in age at diagnosis, but also in the frequency of premenopausal breast cancers when comparing the women from whom we obtained specimens with those for whom we did not. However, these two groups of women were very similar regarding a number of other breast cancer risk factors and tumor characteristics (data published previously25). After taking into account age and year of diagnosis, the participants whose tumors were included in the tissue microarrays were very similar to those for whom we were unable to obtain tissue blocks.
We obtained pathology material for 3752 participants. Of these, 390 specimens were hematoxylin-and-eosin-stained slides only and 45 tissue blocks had to be returned to the lending hospital before construction of the tissue microarrays and thus could not be included. Hematoxylin and eosin sections of the corresponding 3317 paraffin-embedded tissue blocks were reviewed by a single pathologist to confirm the cancer diagnosis, classify the cancer according to histologic type and grade (Nottingham), and circle the area from which the cores for the tissue microarrays would be taken. Pathology review identified 420 tumor blocks as unusable for tissue microarray construction. The majority of exclusions were because the block did not contain residual tumor (60%) or there was insufficient tumor for the tissue microarray (26%). Tissue microarrays were constructed in the Dana-Farber Harvard Cancer Center Tissue Microarray Core Facility, Boston, MA. Three 0.6-mm cores were obtained from each breast cancer and were inserted into the recipient tissue microarray blocks. In total, 23 tissue microarray blocks were constructed from 3093 cancers and positive lymph nodes from 2897 participants. We excluded from the current analysis participants with positive lymph nodes only (n=25), lobular or ductal carcinoma in situ (n=401), and additional rare tumor types including malignant phyllodes tumors, neuroendocrine carcinoma and angiosarcoma (n=10).
Immunohistochemical Analysis
We performed immunohistochemical staining for ER-α, progesterone receptor (PR), HER2, cytokeratin 5/6 and epidermal growth factor receptor (EGFR) on 5 μm paraffin sections cut from the tissue microarray blocks. Immunostains for each marker were performed in a single staining run on a Dako Autostainer (Dako Corporation, Carpinteria, CA, USA). These particular biomarkers were selected for analysis because they have been commonly used as a surrogate to classify invasive breast cancers according to their molecular phenotypes.4, 26, 27, 28, 29 Sources and dilutions of the primary antibodies used in this study are listed in Table 1. The immunostaining protocols for ER-α, PR, HER2, cytokeratin 5/6 and EGFR have been previously described in detail.25 Immunostaining for ER-β was performed on tissue sections following deparaffinization in two 5-min changes of xylene and rehydration through graded alcohols to distilled water. After blocking endogenous peroxidase activity, sections were subjected to heat-induced epitope retrieval in a vegetable steamer in citrate buffer (pH 6.1) for 30 min. Following heat-induced epitope retrieval, the primary monoclonal antibody ER-β1 (clone PPG5/10, Serotec) was applied to the sections at a dilution of 1:50 and the slides were incubated over night at 4°C followed by incubation with the biotinylated universal secondary antibody and the avidin–biotin complex. Visualization was performed with liquid 3,3′-diaminobenzidine as the chromogen substrate. Appropriate positive and negative controls were included in all staining runs.
Immunostained tissue microarray slides were evaluated for ER-α and PR expression, HER2 protein overexpression and expression of cytokeratin 5/6 and EGFR in each core. Tumor cells that showed nuclear staining for ER-α or PR were considered ER+ or PR+, whereas all ER− or PR− cases showed complete absence of tumor cell staining. Of note, low positive ER or PR (1–10% of tumor cell nuclei staining) and positive ER or PR (>10% of tumor cell nuclei staining) were collapsed into a single ER or PR ‘positive’ category for the purposes of this analysis. Tumor cells were considered positive for HER2 protein overexpression when more than 10% of the cells showed moderate or strong membrane staining (2+ and 3+). The results of analyses in which HER2 positivity was defined as 3+ were very similar to those presented with a definition of 2+ and 3+.25 Cases were considered basal cytokeratin positive or EGFR+ if any cytoplasmic and/or membranous staining was detected in the tumor cells, even if focal. These latter criteria are similar to those previously used for scoring these markers in invasive basal-like cancers.4, 26, 27 Tumor cells that showed distinct nuclear staining (regardless of the presence of cytoplasmic staining) were scored as ER-β+ (Figure 1).
Classification of Molecular Phenotype
Immunostained tissue microarray sections were reviewed under a microscope and visually scored for each individual tissue core as described earlier. We classified a case as positive if there was staining in any of the three cores from that case and negative if there was no immunostaining present. Cases that were ER-α+ and/or PR+ and HER2− were classified as luminal A cancers, cases that were ER-α+ and/or PR+ and HER2+ as luminal B cancers, cases that were ER-α−, PR− and HER2+ as HER2 type and cases that were negative for ER-α, PR and HER2 and positive for cytokeratin 5/6 and/or EGFR were categorized as basal-like. Cases that lacked expression of all five markers were considered ‘unclassified’.
Statistical Analysis
χ2-Tests were used to evaluate the independence of selected variables under the null hypothesis. All statistical tests were two sided and P-values <0.05 were considered statistically significant. Breast-cancer-specific survival was calculated from the date of diagnosis to the date of death from breast cancer or the follow-up cutoff. For the estimation of breast-cancer-specific survival, deaths from any other causes were censored. An additional 57 women were excluded due to missing data. The Kaplan–Meier product limit method was used to estimate survival according to ER-α/ER-β status and was compared across groups using the log-rank statistic.
Results
The population for this analysis consisted of invasive breast cancers that developed in women in the Nurses' Health Study after the baseline questionnaire (1976) through the 1996 follow-up cycle that could be classified into one of the four molecular phenotypes and that had evaluable ER-β-stained tissue microarray cores (n=2170). Based on immunostaining data from five of the markers used (ER-α, PR, HER2, EGFR and cytokeratin 5/6), 1585 invasive tumors were classified as luminal A (73%); 115 were luminal B (5%); 125 were HER2 type (6%) and 240 were basal-like (11%). There were also 105 invasive tumors that were considered unclassifiable (ER-α−/PR−/HER2−/EGFR−/cytokeratin 5/6−) for which ER-β staining results were available. An additional 234 invasive cases could not be classified because of non-evaluable staining, or lack of tumor tissue in the core.
Overall, 68% of invasive cancers were ER-β+. Expression of ER-β was significantly associated with expression of ER-α (P<0.0001) and PR (P<0.0001), and was inversely related to overexpression of HER2 (P=0.004), expression of cytokeratin 5/6 (P=0.02) and EGFR (P=0.006). ER-β expression was significantly related to molecular category (P<0.0001) and was more common in luminal A (72%) and luminal B (68%) subtypes than in HER2 or basal-like types (Table 2). However, despite their being defined by the absence of ER-α expression, 55% of HER2 and 60% of basal-like invasive cancers showed expression of ER-β. A similar percentage (48%) of ER-β positivity was seen in unclassified tumors.
The frequency of ER-β among the various histologic types of invasive cancer is shown in Table 3. ER-β expression was seen in all types, including 63% (952/1500), of invasive ductal carcinomas, 87% (200/230) of invasive lobular carcinomas, 83% (30/36) of mucinous carcinomas and 100% (7/7) of tubular carcinomas. Overall, ER-β− tumors at presentation were larger in size (P=0.0008), had a greater extent of lymph node involvement (P=0.06) and were higher grade at the time of diagnosis (P<0.0001) (Table 4). For 2145 cancers with available information on tumor grade, 85% of grade I, 71% of grade II and 49% of grade III cancers showed positive ER-β staining.
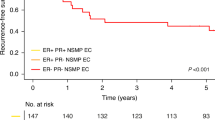
Breast cancer survival cross-classified by ER-α/ER-β status is shown in Figure 2. As expected, patients with ER-α+ tumors had greater survival than patients with ER-α− tumors (P<0.0001). However, overall ER-β status was not associated with improved breast cancer survival (P=0.54). Furthermore, no beneficial effect of ER-β positivity on survival was seen among ER-α+ tumors (P=0.37), ER-α− tumors (P=0.10), or ER-α+ tumors treated with tamoxifen (P=0.55).
Discussion
In this large population-based study, we have shown that 68% of invasive breast cancers express ER-β as detected by a monoclonal antibody to ER-β1. The frequency of ER-β expression among breast cancers in this series is similar to that reported in earlier studies that also used immunohistochemical methods for its detection.11, 12, 14, 18, 21 In addition, we confirmed that there is a subset of ER-α− cancers that express ER-β. Among the ER-α− tumors in this analysis, 56% (279/501) were ER-β+. This finding is virtually identical to that of Skliris et al,23 who reported that 58% of ER-α− tumors were ER-β1+. We found that 55% of cancers coexpress ER-α and ER-β, 22% of cancers expressed ER-α, but were ER-β−, and 13% of cancers were ER-α− and ER-β+. These results are also similar to summary data presented by Skliris et al24 and to the recent findings of Novelli et al10 (Figure 3).
Our understanding of the biology of ER-β and its role in invasive breast cancer remains elusive and results of earlier studies have been somewhat contradictory. ER-β has at least five isoforms (ER-β1-5)30 of which three, ER-β1, ERβ-2 (also referred to as ER-βcx) and ER-β-5, have been identified in breast cancers. Much of the conflicting data in the literature are likely because of the fact that there are various ER-β isoforms and to the complexity of their interactions with ER-α, as well as to the use of a variety of detection methods. Recent gene expression profiling studies have shown that ER-β probably regulates a distinct subset of genes involved in cellular proliferation and apoptosis.15 It is currently believed that ER-α promotes proliferation whereas ER-β is antiproliferative. Accordingly, declining levels of ER-β have been reported to be associated with an increased risk of progression to invasive cancer.16, 17, 31 We found that ER-β− tumors were of higher grade, larger size, more likely to have lymph node involvement and of higher stage at time of diagnosis, all of which support a potential antiproliferative role for ER-β.
The prognostic and predictive importance of ER-β expression has been somewhat controversial, again in large part because of the issues mentioned above. Recently, however, there has been renewed interest in ER-β and its potential clinical relevance.6, 7, 10, 13, 24 A recent study by Honma et al13 examined ER-β expression in invasive breast cancers from 442 Japanese women, all of whom were treated with adjuvant tamoxifen. In that study positive immunohistochemical staining for ER-β1, using the PPG5/10 clone, was associated with a significantly increased disease-free survival and overall survival in tamoxifen-treated women. Of particular interest, this response was also seen in ER-α− tumors; a finding in agreement with earlier studies.6, 32 For example, Gruvberger-Saal et al32 also noted better distant disease-free survival in women treated with tamoxifen who had ER-β+/ER-α− cancers. Two recent studies have provided additional insight into the potential prognostic significance of ER-β. In the first, Novelli et al10 reported that ER-β positivity was associated with a more aggressive clinical course among node-positive breast cancer patients. In contrast, these authors found that ER-β1 positivity predicted favorable response to endocrine therapy among lymph node-negative patients. It should be noted that the patients in the Novelli study were treated with a variety of adjuvant chemotherapy regimens so differences in outcome must be interpreted with caution. Nonetheless, these results suggest that ER-β may potentially be a useful prognostic and/or predictive factor among women with lymph-node-negative breast cancer. In the second study, Shaaban et al7 reported that ER-β2 was a powerful prognostic indicator in women with breast cancer. Furthermore, these authors found that nuclear and cytoplasmic expression of ER-β2 differentially affected outcome.7 Again though the possibility that differences in systemic therapies may have affected outcome is not addressed in this paper.
In the present study, ER-β positivity did not appear to have a beneficial effect on survival regardless of ER-α status. This lack of association with ER-β and improved survival seen in earlier studies may also be the result of considerable treatment variability; an inherent limitation in this large population of women treated over a long period of time. However, even when we examined survival in women with ER-α+ tumors treated with tamoxifen, ER-β positivity was still not associated with better outcome. Therefore, we were unable to further clarify the predictive and prognostic value of ER-β1.
Estrogen receptor-β expression was observed in each of the molecularly defined phenotypes of invasive breast cancer, even among those defined by the absence of ER-α expression, ie, the HER2 and basal-like subtypes. The association between ER-β and HER2 overexpression is unclear. Some studies have shown a positive association with HER2 overexpression11, 33, 34 whereas others have shown an inverse relationship.23, 32 Cytokeratin 5/6, a marker of the basal-like subtype of breast cancer, was only weakly correlated with ER-β in one study.23 In such ER-α−/ER-β+ tumors, it has been proposed that one or more of the ER-β isoforms are actually promoting proliferation, and this is supported by the frequent coexpression of ER-β with the proliferation markers Ki-6714, 23, 33 and topoisomerase IIα.11 In this study, ER-β was inversely related to HER2 expression as well as to the expression of the basal markers cytokeratin 5/6 and EGFR, in keeping with the findings of previous studies.23, 32
Our study has several potential limitations. First, we were unable to obtain tissue blocks from all breast cancers arising in this cohort. Our success in doing so was highly correlated with time between diagnosis and initiation of our tissue block collection. After taking into account the effect of age and year of diagnosis, the women for whom we were able to obtain tumor specimens were very similar to those for whom we were unable to obtain specimens.25 In addition, the frequency of ER-α and ER-β positivity among invasive tumors was very similar to other populations suggesting that samples included in this study are representative of the overall US population. Second, we used immunohistochemical markers as a surrogate to classify breast cancers into the molecular phenotypes defined by expression profiles. Although the antibody panel we used in this study has been shown to be a reliable proxy for classification of invasive breast cancers categorized by gene expression,4, 26, 27, 28, 29 the correlation is not perfect and there will be some misclassification of these phenotypes. The categories as defined by the immunohistochemical markers have been shown to be associated with prognostic markers and survival consistent with what has been seen with classification based on RNA expression assays, suggesting that both methods are capturing distinct subgroups.26, 35 Misclassification of phenotypes may underestimate true differences between the subtypes. Finally, there is considerable variability in the specificity of commercially available ER-β antibodies36 and caution should be used when directly comparing results among studies. In addition to there being several ER-β isoforms as discussed above, technical aspects such as fixation protocols and antigen retrieval methods could explain varying results. We used the Serotec PPG5/10 clone, an antibody directed against ER-β1, and a standard protocol as described above. The PPG5/10 clone has previously been validated by western blot analysis17, 20, 31 and when compared with other commercially available ER-β antibodies, the PPG5/10 clone showed consistent strong nuclear staining and has been well validated in a number of studies at this point.7, 13, 36 Moreover, ER-β1 is the only fully functional isoform.37 As a result, it has been considered a favored antibody for ER-β detection.38
In summary, in this large population-based study, ER-β expression was commonly seen in luminal A and B types of invasive breast cancer. Furthermore, expression of ER-β was also seen in a subset of HER2 and basal-like cancers, which are considered to be hormone-receptor-negative breast cancers. The potential role of ER-β in the development and progression of invasive breast cancers and as a prognostic and predictive factor in breast cancers defined by lack of expression of ER-α expression merits further investigation.
References
Perou CM, Jeffrey SS, van de Rijn M, et al. Distinctive gene expression patterns in human mammary epithelial cells and breast cancers. Proc Natl Acad Sci USA 1999;96:9212–9217.
Sorlie T, Perou CM, Tibshirani R, et al. Gene expression patterns of breast carcinomas distinguish tumor subclasses with clinical implications. Proc Natl Acad Sci USA 2001;98:10869–10874.
Sorlie T, Tibshirani R, Parker J, et al. Repeated observation of breast tumor subtypes in independent gene expression data sets. Proc Natl Acad Sci USA 2003;100:8418–8423.
Brenton JD, Carey LA, Ahmed AA, et al. Molecular classification and molecular forecasting of breast cancer: ready for clinical application? J Clin Oncol 2005;23:7350–7360.
Kuiper GG, Enmark E, Pelto-Huikko M, et al. Cloning of a novel receptor expressed in rat prostate and ovary. Proc Natl Acad Sci USA 1996;93:5925–5930.
Hodges-Gallagher L, Valentine CD, El Bader S, et al. Estrogen receptor beta increases the efficacy of antiestrogens by effects on apoptosis and cell cycling in breast cancer cells. Breast Cancer Res Treat 2008;109:241–250.
Shaaban AM, Green AR, Karthik S, et al. Nuclear and cytoplasmic expression of ERbeta1, ERbeta2, and ERbeta5 identifies distinct prognostic outcome for breast cancer patients. Clin Cancer Res 2008;14:5228–5235.
Borgquist S, Holm C, Stendahl M, et al. Oestrogen receptors alpha and beta show different associations to clinicopathological parameters and their co-expression might predict a better response to endocrine treatment in breast cancer. J Clin Pathol 2008;61:197–203.
Hopp TA, Weiss HL, Parra IS, et al. Low levels of estrogen receptor beta protein predict resistance to tamoxifen therapy in breast cancer. Clin Cancer Res 2004;10:7490–7499.
Novelli F, Milella M, Melucci E, et al. A divergent role for estrogen receptor-beta in node-positive and node-negative breast cancer classified according to molecular subtypes: an observational prospective study. Breast Cancer Res 2008;10:R74.
Nakopoulou L, Lazaris AC, Panayotopoulou EG, et al. The favourable prognostic value of oestrogen receptor beta immunohistochemical expression in breast cancer. J Clin Pathol 2004;57:523–528.
Mann S, Laucirica R, Carlson N, et al. Estrogen receptor beta expression in invasive breast cancer. Hum Pathol 2001;32:113–118.
Honma N, Horii R, Iwase T, et al. Clinical importance of estrogen receptor-beta evaluation in breast cancer patients treated with adjuvant tamoxifen therapy. J Clin Oncol 2008;26:3727–3734.
Jensen EV, Cheng G, Palmieri C, et al. Estrogen receptors and proliferation markers in primary and recurrent breast cancer. Proc Natl Acad Sci USA 2001;98:15197–15202.
Chang EC, Frasor J, Komm B, et al. Impact of estrogen receptor beta on gene networks regulated by estrogen receptor alpha in breast cancer cells. Endocrinology 2006;147:4831–4842.
Roger P, Sahla ME, Makela S, et al. Decreased expression of estrogen receptor beta protein in proliferative preinvasive mammary tumors. Cancer Res 2001;61:2537–2541.
Shaaban AM, O'Neill PA, Davies MP, et al. Declining estrogen receptor-beta expression defines malignant progression of human breast neoplasia. Am J Surg Pathol 2003;27:1502–1512.
Skliris GP, Carder PJ, Lansdown MR, et al. Immunohistochemical detection of ERbeta in breast cancer: towards more detailed receptor profiling? Br J Cancer 2001;84:1095–1098.
Omoto Y, Inoue S, Ogawa S, et al. Clinical value of the wild-type estrogen receptor beta expression in breast cancer. Cancer Lett 2001;163:207–212.
Saunders PT, Millar MR, Williams K, et al. Expression of oestrogen receptor beta (ERbeta1) protein in human breast cancer biopsies. Br J Cancer 2002;86:250–256.
Fuqua SA, Schiff R, Parra I, et al. Estrogen receptor beta protein in human breast cancer: correlation with clinical tumor parameters. Cancer Res 2003;63:2434–2439.
Poola I, Fuqua SA, De Witty RL, et al. Estrogen receptor alpha-negative breast cancer tissues express significant levels of estrogen-independent transcription factors, ERbeta1 and ERbeta5: potential molecular targets for chemoprevention. Clin Cancer Res 2005;11:7579–7585.
Skliris GP, Leygue E, Curtis-Snell L, et al. Expression of oestrogen receptor-beta in oestrogen receptor-alpha negative human breast tumours. Br J Cancer 2006;95:616–626.
Skliris GP, Leygue E, Watson PH, et al. Estrogen receptor alpha negative breast cancer patients: estrogen receptor beta as a therapeutic target. J Steroid Biochem Mol Biol 2008;109:1–10.
Tamimi RM, Baer HJ, Marotti J, et al. Comparison of molecular phenotypes of ductal carcinoma in situ and invasive breast cancer. Breast Cancer Res 2008;10:R67.
Nielsen TO, Hsu FD, Jensen K, et al. Immunohistochemical and clinical characterization of the basal-like subtype of invasive breast carcinoma. Clin Cancer Res 2004;10:5367–5374.
Abd El-Rehim DM, Ball G, Pinder SE, et al. High-throughput protein expression analysis using tissue microarray technology of a large well-characterised series identifies biologically distinct classes of breast cancer confirming recent cDNA expression analyses. Int J Cancer 2005;116:340–350.
Abd El-Rehim DM, Pinder SE, Paish CE, et al. Expression of luminal and basal cytokeratins in human breast carcinoma. J Pathol 2004;203:661–671.
van de Rijn M, Perou CM, Tibshirani R, et al. Expression of cytokeratins 17 and 5 identifies a group of breast carcinomas with poor clinical outcome. Am J Pathol 2002;161:1991–1996.
Zhao C, Dahlman-Wright K, Gustafsson JA . Estrogen receptor beta: an overview and update. Nucl Recept Signal 2008;6:e003.
Skliris GP, Munot K, Bell SM, et al. Reduced expression of oestrogen receptor beta in invasive breast cancer and its re-expression using DNA methyl transferase inhibitors in a cell line model. J Pathol 2003;201:213–220.
Gruvberger-Saal SK, Bendahl PO, Saal LH, et al. Estrogen receptor beta expression is associated with tamoxifen response in ERalpha-negative breast carcinoma. Clin Cancer Res 2007;13:1987–1994.
Choi Y, Pinto M . Estrogen receptor beta in breast cancer: associations between ERbeta, hormonal receptors, and other prognostic biomarkers. Appl Immunohistochem Mol Morphol 2005;13:19–24.
Umekita Y, Enokizono N, Sagara Y, et al. Immunohistochemical studies on oncogene products (EGF-R, c-erbB-2) and growth factors (EGF, TGF-alpha) in human breast cancer: their relationship to oestrogen receptor status, histological grade, mitotic index and nodal status. Virchows Arch A Pathol Anat Histopathol 1992;420:345–351.
Carey LA, Perou CM, Livasy CA, et al. Race, breast cancer subtypes, and survival in the Carolina Breast Cancer Study. JAMA 2006;295:2492–2502.
Skliris GP, Parkes AT, Limer JL, et al. Evaluation of seven oestrogen receptor beta antibodies for immunohistochemistry, western blotting, and flow cytometry in human breast tissue. J Pathol 2002;197:155–162.
Leung YK, Mak P, Hassan S, et al. Estrogen receptor (ER)-beta isoforms: a key to understanding ER-beta signaling. Proc Natl Acad Sci USA 2006;103:13162–13167.
Carder PJ, Murphy CE, Dervan P, et al. A multi-centre investigation towards reaching a consensus on the immunohistochemical detection of ERbeta in archival formalin-fixed paraffin embedded human breast tissue. Breast Cancer Res Treat 2005;92:287–293.
Acknowledgements
Funding for this research was provided by GlaxoSmithKline (WE234, EPI40307), Public Health Service Grant CA087969 and SPORE in Breast Cancer Grant CA089393, from the National Cancer Institute, National Institutes of Health and Department of Health and Human Services.
Author information
Authors and Affiliations
Corresponding author
Additional information
Disclosure/conflict of interest
The authors declare no conflict of interest.
Rights and permissions
About this article
Cite this article
Marotti, J., Collins, L., Hu, R. et al. Estrogen receptor-β expression in invasive breast cancer in relation to molecular phenotype: results from the Nurses' Health Study. Mod Pathol 23, 197–204 (2010). https://doi.org/10.1038/modpathol.2009.158
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/modpathol.2009.158
Keywords
This article is cited by
-
PES1 promotes the occurrence and development of papillary thyroid cancer by upregulating the ERα/ERβ protein ratio
Scientific Reports (2019)
-
In breast cancer subtypes steroid sulfatase (STS) is associated with less aggressive tumour characteristics
British Journal of Cancer (2018)
-
The presence and impact of estrogen metabolism on the biology of triple-negative breast cancer
Breast Cancer Research and Treatment (2017)
-
Agonists and knockdown of estrogen receptor β differentially affect invasion of triple-negative breast cancer cells in vitro
BMC Cancer (2016)
-
Expression of matrix metalloproteinases and their inhibitors in different immunohistochemical-based molecular subtypes of breast cancer
BMC Cancer (2014)