Abstract
Peritubular capillary C4d staining in allograft kidney is an important criterion for antibody-mediated rejection. Whether BK virus infection can result in complement activation is not known. We studied 113 renal allograft biopsies from 52 recipients with a history of BK virus activation. The samples were classified into four groups according to the concurrent detection of BK virus DNA in urine, plasma, and/or biopsy: BK-negative (n=37), viruria (n=53), viremia (n=7), and nephropathy (n=16) groups. The histological semiquantitative peritubular capillary C4d scores in the viremia (0.3±0.8) and BK nephropathy (0.6±0.9) groups were lower than those in the BK-negative group (1.2±1.1, P=0.05 and P=0.06, respectively) and the viruria group (1.2±1.1, P=0.04 and P=0.06, respectively). Diffuse or focal peritubular capillary C4d staining was present in 9/76 (12%) and 14/76 (19%) of all samples with concurrent BK virus reactivation (viruria, viremia, and nephropathy). The diagnosis of antibody-mediated rejection could be established in 7/9 (78%) and 5/14 (36%) of these samples, respectively. Diffuse tubular basement membrane C4d staining was restricted to BK nephropathy cases (4/16, 25%). Semiquantitative tubular basement membrane C4d scores were higher in BK nephropathy (1.2±1.3) compared with BK-negative (0.05±0.3, P=0.017) and viruria (0.0±0.0, P=0.008) groups. Bowman's capsule C4d staining was more frequent in BK nephropathy (5/16) compared with the aforementioned groups (2/36 (P=0.023) and 4/51 (P=0.03), respectively). Within the BK nephropathy group, samples with tubular basement membrane stain had more infected tubular epithelial cells (12.1±7.6% vs 4.4±5.0%, P=0.03) and a trend toward higher interstitial inflammation scores. In conclusion, peritubular capillary C4d staining remains a valid marker for the diagnosis of antibody-mediated rejection in the presence of concurrent BK virus infection. A subset of biopsies with BK nephropathy shows tubular basement membrane C4d staining, which correlates with marked viral cytopathic effect.
Similar content being viewed by others
Main
BK virus is a human polyomavirus that can be associated with premature graft failure due to BK virus nephropathy in renal transplant immunosuppressed recipients.1, 2, 3, 4, 5, 6 Early diagnosis is the key to reduce the incidence of disease, because advanced nephropathy does not respond neither to reducing immunosuppression nor to antiviral drugs, such as cidofovir, leflunomide, or fluoroquinolones.7 For this reason, serial urinary and plasma measurements of BK virus DNA using real-time quantitative PCR are used to monitor reactivation of BK virus, which is widely latent in the kidney of healthy subjects and transplant recipients.
C4d is a classical pathway complement degradation product that, when detected in peritubular capillaries, correlates with antibody-mediated rejection, and is associated with poor renal allograft outcome.8, 9, 10, 11 It is believed that the activation of C4 by antigen–antibody interaction results in its cleavage to C4a and C4b. C4b binds to amino or hydroxyl groups and is then converted to C4d, which can covalently bind with endothelial basement membranes in peritubular capillaries.12
In vitro experiments with purified Epstein–Barr virus and cytomegalovirus-infected fibroblasts have shown that viruses can directly cause activation of serum complement.13, 14 This notion has not been directly tested for BK virus. Honsová et al.15 described focal peritubular capillary C4d staining in 9/12 (75%) cases of BK nephropathy, but did not measure the presence of donor-specific alloantibodies in their patients. In contrast, Ott et al.16 observed focal peritubular capillary C4d staining in only 2/6 (33%) of biopsies showing BK nephropathy, whereas Meehan et al.17 found no staining in any of the 28 BK nephropathy biopsies studied by them. Bracamonte et al.18 and Hever et al.19 showed immune complex deposits in the tubular basement membrane in a subset of BK nephropathy patients, but did not perform immunohistochemistry for C4d in their biopsy material. In this study we systematically analyzed C4d staining in 113 biopsies from 52 renal transplant recipients with BK virus activation. The results of immunohistochemistry were correlated with clinical and pathological findings.
Materials and methods
Design
We retrospectively reviewed clinical information and surgical pathology findings in 113 biopsies from 52 patients with a history of BK virus activation defined as at least one post-transplant episode of viremia and/or viruria. All the studied samples were ‘for-cause’ biopsies performed for rising serum creatinine level between 2004 and 2007. Concurrent BK virus activation was defined as the presence of BK nephropathy, or the detection of BK virus DNA in the urine or plasma within 14 days of performing the biopsy. BK nephropathy was defined as the presence of intratubular viral inclusions with a positive in situ hybridization test for viral DNA.6 This study was conducted in accordance with the procedures established by the University of Pittsburgh institutional review board (IRB protocol no. 0602155).
Histological Evaluation
Semiquantitative histological scores for acute rejection components, namely glomerulitis (g), interstitial inflammation (i), tubulitis (t), and intimal arteritis (v), were recorded. Similarly, pathological scoring for chronic changes, such as chronic transplant glomerulopathy (cg), interstitial fibrosis (ci), and tubular atrophy (ct), was also semiquantitatively assessed using the Banff 1997 criteria for renal allograft pathology.20 In addition, samples were classified as negative, suspicious, or diagnostic for acute antibody-mediated rejection based on the Banff 2001 criteria.11 In BK nephropathy cases, biopsy viral load was assessed using in situ hybridization for BK virus DNA as follows: if the number of positive cells was less than 5, the percentage was estimated as <1%. Otherwise, the approximate percentage of positive tubular cells was rounded to the nearest 5%.
C4d Staining
The standard practice in our medical center is to submit biopsies fixed in formalin for histological evaluation. We routinely performed C4d immunostaining using a rabbit anti-human C4d polyclonal antibody (ALPCO Diagnostics, Windham, NH, USA). In brief, 4 μm, formalin-fixed, paraffin-embedded sections of kidneys were deparaffinized, placed in a pressure cooker for 20 min, and sequentially incubated at 37 °C with TRIS-EDTA (pH 8.5), with 1:50 dilution of primary antibody for 44 min, 1:100 dilution of a biotinylated anti-rabbit secondary antibody for 8 min, streptavidin–alkaline phosphate conjugate for 8 min, Fast red or brown A-naphthol for 8 min, and Fast red or brown B-naphthol for 8 min. The sections were then counterstained with hematoxylin. All reagents were components of the Ventana enhanced alkaline phosphatase red or brown detection kit (Cat no. 760-031, Ventana Medical Systems, Tucson, AZ, USA).
C4d Stain Interpretation
Peritubular capillary staining was classified as diffuse when staining was present in more than half of the microscopic fields examined. Staining was referred to as focal or minimal when present in 10–50% or <10% of the microscopic fields, respectively. The absence of detectable staining was regarded as negative. As immunohistochemical C4d was performed on paraffin-embedded tissue, both focal and diffuse peritubular capillary staining patterns were regarded as significant. This was in accordance with the Banff 2007 recommendations.21 This was also consistent with our observation that, when performed on paraffin-embedded tissue, both diffuse and focal C4d staining patterns were significantly associated with the presence of circulating donor-specific antibodies.22, 23 In individual tubules, staining was considered to be present if more than half of the tubular basement membrane circumference was stained. Staining was further characterized as diffuse, focal, or minimal depending on whether >50%, 10–50%, or <10% of the microscopic fields showed the presence of tubular basement membrane C4d staining, respectively. The absence of detectable stain was regarded as negative. To facilitate statistical analysis, C4d staining in the peritubular capillaries and tubular basement membranes was also assigned a numerical score of 0 (negative), 1 (minimal), 2 (focal), or 3 (diffuse). Bowman's capsule staining was qualitatively recorded as present or absent.
Circulating Donor-Specific Antibodies
Blood samples were collected on the day of transplantation and at multiple times after transplant. Serum/plasma specimens were tested using enzyme-linked immunosorbent assay (ELISA) or multiplex bead array (Luminex). For ELISA, we used commercial LAT-M and LAT-1288 ELISA kits (One Lambda, Canoga Park, CA, USA) to identify IgG anti-HLA class I and class II specific alloantibodies. The assay was read at 630 nm using an ELISA reader (ELX 800, Bio-Tek, Winooski, Vermont, USA, and computer software from One Lambda). All tests were run in duplicate and the results of ELISA screen were expressed as a percentage of the average positive serum control. For Luminex, antibody specificity was determined using single antigen beads supplied by two commercial vendors (One Lambda, Inc., and Tepnel Life Codes Corporation, Stamford, CT, USA). The procedure was performed in accordance with the manufacturer's instructions to identify IgG anti-HLA class I and class II specific alloantibodies. The presence of circulating donor-specific antibodies was determined based on detection of antibodies within a 3-month period of the biopsy date.
BK Virus DNA in Urine and Plasma
The urine and plasma samples were tested for BK virus DNA load using an in-house assay developed in the clinical laboratory.24 Both urine and plasma samples are usually assessed in the same day.
In brief, blood samples were collected in EDTA tubes. Centrifugation was used for plasma but not for urine samples. The samples were frozen (−80 °C) within 6 h and used within 7 days. QIAamp Blood maxikit (catalog no. 51192; Qiagen) and QIAamp Blood minikit (catalog no. 51104; Qiagen) were used to extract DNA from 5 ml of urine or 200 μl of plasma. Oligonucleotide sequences, derived from the BK virus (Dunlop strain; GenBank accession number NC001538) capsid protein-1 (VP-1) gene, were amplified using published reaction conditions. The results were calculated from standard curves and were expressed as copies of viral DNA per ml.
Statistics
Descriptive statistical values were presented as mean±s.d. Continuous values were compared using analysis of variance (Kruskal–Wallis one-way analysis of variance on ranks and Mann–Whitney rank-sum test). Categorical values were compared using Fisher's exact test. All analyses were performed using Sigma Stat 2.0.3 software (SPSS Inc., Chicago, IL, USA), and P-values <0.05 were considered statistically significant.
Results
Clinical Features
We studied 113 biopsies from 52 patients with BK virus reactivation (39 males, 13 females). The patient age ranged between 12 and 78 years (mean±s.d.=51.3±16.3). Most patients received lymphocyte depletion therapy at the time of transplant and tacrolimus monotherapy after transplantation. The mean whole blood tacrolimus levels and serum creatinine at the time of biopsies were 8.3±4.6 ng/ml and 2.8±1.31 mg per 100 ml, respectively.
The samples were divided into four groups according to the concurrent detection of BK virus DNA in urine, plasma, and biopsies using quantitative PCR and in situ hybridization: (1) BK-negative group when BK virus DNA was absent in plasma, urine, and biopsy (n=37); (2) viruria when viral DNA was detected in urine but not in plasma or biopsy (n=53); (3) viremia when BK virus DNA was present in the plasma with no detectable viral cytopathic effects and negative BK in situ hybridization in biopsy tissue (n=7); and (4) BK nephropathy when BK virus DNA was detected in the biopsy (n=16) (Table 1). BK nephropathy cases included in-house (n=7) as well as consultation cases (n=9), for which no PCR data were available. All the in-house BK nephropathy samples had concurrent detectable BK virus DNA in urine (range 2.92E+06 to 2.3E+10 copies/ml), and all but one had detectable viral DNA in plasma (range 1.54E+03 to 5.98E+05 copies/ml). These four patient groups (BK negative, viruria, viremia, and nephropathy) showed no significant differences in age, sex, whole blood tacrolimus levels, or post-transplant time of initial evaluation (Table 1). Serum creatinine in viremia samples (3.5±1.1 mg per 100 ml) was significantly higher compared with BK-negative (2.8±1.5 mg per 100 ml, P=0.024) and viruria (2.6±1.1 mg per 100 ml, P=0.037) samples, and tended to be higher than BK nephropathy samples, although serum creatinine values were not available in 6/16 consultation biopsies with nephropathy (P=0.08) (Table 1).
Histological Evaluation
Biopsies were performed 787±708 days after transplantation. These biopsies were graded according to Banff 97 criteria20 for acute rejection as follows: no rejection (n=18), borderline changes suspicious for acute rejection (n=26), IA (n=32), IB (n=18), IIA (n=3), and BK nephropathy (n=16). Histological evaluation showed a higher score of interstitial inflammation in BK nephropathy (2.4±0.6) compared with BK-negative (1.6±0.9, P=0.002) and BK viruria (1.5±0.9, P<0.001) groups (Table 2). The tubulitis score was also proportionately higher in BK nephropathy (2.5±0.7) compared with both BK-negative and viruria samples (1.6±1.0, P=0.003 and 1.7±1.4, P=0.004), respectively. No difference was found in the severity of glomerulitis, interstitial fibrosis, or tubular atrophy between these different groups. The progression of interstitial fibrosis was seen in three out of eight patients with BK nephropathy who had follow-up biopsies, and all three patients lost their grafts (960±360 days later).
Peritubular Capillary Staining for C4d
Diffuse peritubular capillary staining was not observed in BK nephropathy or BK viremia groups, and was limited to viruria [9/53 (17%)] and BK-negative [4/37 (11%)] groups (Table 3). Focal staining was observed in 4/16 (25%), 1/7 (14%), 9/53 (17%), and 13/37 (35%) of BK nephropathy, viremia, viruria, and BK-negative samples, respectively. Minimal staining was seen in 1/16 (6%), 0/7 (0%), 16/53 (30%), and 6/37 (16%) of BK nephropathy, viremia, viruria, and BK-negative biopsies, respectively. Semiquantitative peritubular capillary C4d score was lower in BK viremia (0.3±0.8) compared with viruria (1.2±1.1, P=0.04) samples, and tended to be lower than that of BK-negative samples (1.2±1.1, P=0.05). A trend toward a lower peritubular capillary C4d score was detected in the BK nephropathy group (0.6±0.9) compared with the BK-negative (P=0.06) and viruria (P=0.06) groups (Table 3). When all samples with concurrent BK virus reactivation were considered (viruria, viremia, and BK nephropathy, n=76), diffuse and focal peritubular capillary C4d staining were present in 9/76 (12%) and 14/76 (19%) of biopsies, respectively (Table 3). According to the Banff 2001 criteria,11 these were associated with the diagnosis of acute antibody-mediated rejection in 7/9 (78%) and 5/14 (36%) of cases, respectively.
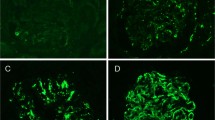
The information on circulating donor-specific antibodies was available for 42 patients at 97 time points (37 BK-negative, 48 viruria, 7 viremia, and 5 in-house BK nephropathy samples). In the BK-negative group, diffuse, focal, minimal, and negative staining were associated with circulating donor-specific antibodies in 3/4 (75%), 7/13 (54%), 1/6 (17%), and 2/14 (14%) specimens, respectively (Table 4). In the viruria group, diffuse (Figure 1), focal, minimal, and negative staining was associated with circulating donor-specific antibodies in 7/8 (88%), 4/8 (50%), 7/14 (50%), and 7/18 (39%) specimens, respectively. In the viremia group, no diffuse peritubular capillary staining was found. One biopsy with focal C4d was associated with circulating donor-specific antibodies. For BK nephropathy, information regarding circulating donor-specific antibodies was available only for five in-house biopsies that showed focal (n=1) or negative (n=4) C4d staining in peritubular capillaries. Of these, only one biopsy was associated with detectable circulating donor-specific antibodies, and this sample did not show any peritubular capillary staining. Thus, no patient with BK nephropathy had a definitive diagnosis of antibody-mediated rejection. The association of different patterns of peritubular capillary C4d staining with the presence of circulating donor-specific antibodies was then compared between these different groups (BK-negative, viruria, viremia, and BK nephropathy). No significant difference was detected. When only samples with available donor-specific antibodies information were considered, it became evident that circulating donor-specific antibodies were absent in only 1/8 (12%) and 5/10 (50%) of all samples with concurrent BK activation with diffuse and focal peritubular capillary staining, respectively (Table 4).
Peritubular Capillary C4d Staining in Previous Biopsies
We then evaluated the prevalence of acute rejection episodes in previous biopsies from patients with concurrent BK virus activation. Previous biopsies were not available for patients with concurrent diffuse C4d stain, whereas they were available for 7, 7, and 11 patients with focal, minimal, and negative C4d stain, respectively. Previous episodes of rejection associated with positive peritubular capillary C4d stain were detected in 4/7 (57%), 3/7 (43%), and 4/11 (36%) of patients with concurrent focal, minimal, and negative C4d stain, respectively. When the number of previous episodes with C4d-positive rejection was compared in these aforementioned groups, no significant difference was detected.
C4d Staining in Tubular Basement Membranes and Bowman's Capsule
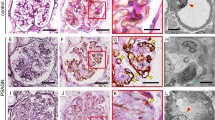
Diffuse tubular basement membrane staining was restricted to BK nephropathy group (4/16 (25%); Table 3). Focal staining was detected in 2/16 (13%) of BK nephropathy biopsies and only in 1/37 (3%) samples within the BK-negative group. It was not encountered in viremia or viruria groups. Minimal staining was observed twice in viremia group (2/7 (29%)) and in one biopsy with BK nephropathy (1/16 (6%)). Semiquantitative tubular basement membrane C4d scores for BK-negative, viruric, viremic, and BK nephropathy groups were 0.05±0.3, 0±0, 0.3±0.5, and 1.2±1.3, respectively. Tubular basement membrane staining scores derived from BK nephropathy biopsies (Figure 2a) were higher compared with both BK-negative (P=0.017) and viruria groups (P=0.008) (Table 3). It is worth mentioning that tubular basement membrane staining was detected in proximal as well as distal tubules, and was not limited to the tubules showing viral cytopathic effects. The proportion of biopsies showing Bowman's capsule staining for C4d (Figure 2b) was also significantly higher in BK nephropathy (5/16) compared with both BK-negative (2/36, P=0.023) and BK viruria groups (4/51, P=0.03).
As tubular basement membrane C4d staining was almost restricted to a subset of biopsies with BK nephropathy, we decided to compare histological parameters in biopsies with (n=7) and without (n=9) such staining (Table 5). Samples with tubular basement membrane C4d stain showed a trend toward more interstitial inflammation (2.7±0.5 with tubular basement membrane staining vs 2.1±0.6 without tubular basement membrane C4d staining; P=0.09), and significantly higher score for viral cytopathic effect (12.1±7.6% with tubular basement membrane C4d staining vs 4.4±5.0% without tubular basement membrane staining; P=0.03) (Table 5).
Clinical Course of Patients with BK Virus Infection and Antibody-Mediated Rejection
None of the patients with BK nephropathy was concurrently diagnosed with antibody-mediated rejection. This diagnosis was made in one patient with viremia, who had focal peritubular capillary C4d staining, and a previous episode of BK nephropathy. Despite cidofovir therapy, this patient developed graft failure 380 days later due to chronic allograft nephropathy. Within the viruria group, 11 samples from 6 patients were diagnosed with antibody-mediated rejection (diffuse peritubular capillary stain (n=3) and focal stain (n=3)). Two of the three patients with diffuse C4d stain developed graft failure 239±113 days later because of due to chronic allograft nephropathy, whereas the third patient had functioning graft at the last follow-up (1018 days later). One of the three patients with focal C4d staining had graft failure owing to primary allograft dysfunction (182 days later), whereas the other two had functioning graft at the last follow-up (1120±303 days later).
Discussion
BK virus is widely latent in the kidney. Immunosuppression leads to viral reactivation manifested as asymptomatic viruria, which may progress to viremia or frank nephropathy. Two viral antigens, namely VP-1 and Large T antigen, are known to elicit antibody response in humans, and it is conceivable that antigen–antibody interactions may activate the complement pathway. Indeed, tubular basement membrane immune complex deposits have been shown in approximately 50% of BK nephropathy patients by immunofluorescence examination and electron microscopy (Bracamonte et al.18 (16/30, 53%) and Hever et al.19 (13/26, 50%)). However, neither of the aforementioned studies specifically looked for C4d deposits within the immune complexes.
It is important to ascertain whether BK virus reactivation can result in C4d staining, as the presence of C4d in peritubular capillaries is now a widely accepted criterion for making the diagnosis of antibody-mediated rejection. Certainly, there is precedence in the literature for other viruses such as cytomegalovirus and Epstein–Barr virus, resulting in complement activation.13, 14 However, the question has not received sufficient attention in the case of BK virus. Honsová et al.15 reported that BK nephropathy was associated with focal peritubular capillary C4d staining in 9/12 (75%) needle biopsy samples, but did not present any data on the presence or absence of donor-specific antibodies. In our study, focal C4d stain was observed in only 4/16 (25%) of our biopsies with BK nephropathy. Peritubular capillary C4d scores were lower in viremia cases and tended to be lower in BK nephropathy cases compared with viruria and BK-negative cases. This argues strongly against BK virus activation being a cause of peritubular capillary C4d staining in the allograft kidney, particularly as peritubular capillary endothelial cells are not even a known target for BK virus infection. The lower peritubular capillary C4d scores in BK nephropathy and viremia might be expected, because both these clinical conditions are associated with excessive immunosuppression.
The presence of diffuse (n=9) and focal (n=14) peritubular capillary C4d staining in patients with concurrent BK virus reactivation (including viruria, viremia, and nephropathy) was highly specific for the presence of circulating donor-specific antibodies, which were detected in 7/8 (88%) and 5/10 (50%) of such samples, respectively. These were associated with the diagnosis of acute antibody-mediated rejection in 7/9 (78%) and 5/14 (36%) of cases, respectively. This observed association with donor-specific antibodies (diffuse 88 and focal 50%) is comparable to what we previously observed in biopsies taken from patients with acute cellular rejection not selected for BK virus activation (diffuse 94%, focal 38%).23 Thus, the presence of peritubular capillary C4d stain should alert the pathologist to the possibility of antibody-mediated rejection, even if there is concomitant active BK virus infection.
In contrast to the peritubular capillary C4d score, tubular basement membrane C4d score was significantly higher in BK nephropathy samples compared with both BK-negative and viruric groups. This suggests that BK virus infection of the renal tubules might activate the complement pathway and result in tubular basement membrane C4d staining. Within the BK nephropathy group, tubular basement membrane C4d staining was associated with a higher viral load within the tubular epithelial compartment. This was in concordance with previous studies that showed that immune complex deposits in the tubular basement membrane are associated with more severe infection.18, 19 In our experience, tubular basement membrane staining for C4d in paraffin-embedded tissue is an uncommon phenomenon that is seen in <5% of the allograft biopsies.22 This staining pattern may not be entirely specific to BK nephropathy because we have seen it occasionally in the absence of active BK virus infection in patients with acute cellular rejection and in one case of recurrent antiglomerular basement membrane glomerulonephritis.25 However, its presence, especially when accompanied by Bowman's capsule C4d staining, should prompt a more careful examination of the biopsy for viral inclusions. It has been previously shown that BK virus can infect the Bowman's capsular epithelium in 20% of the biopsies with BK nephropathy.26
In summary, although the number of patients studied is limited, it seems that BK virus replication is not per se associated with complement deposition in the peritubular capillaries. Hence, peritubular capillary C4d remains a valid marker for antibody-mediated rejection in patients with BK virus reactivation. However, a subset of BK nephropathy patients show C4d staining in the tubular basement membrane and, less frequently, in the Bowman's capsule. These patients have more tubular viral cytopathic effect than patients without C4d staining in the tubular basement membrane.
Accession codes
References
Ahuja M, Cohen EP, Dayer AM, et al. Polyoma virus infection after renal transplantation. Use of immunostaining as a guide to diagnosis. Transplantation 2001;71:896–899.
Hurault de Ligny B, Etienne I, Francois A, et al. Polyomavirus-induced acute tubulo-interstitial nephritis in renal allograft recipients. Transplant Proc 2000;32:2760–2761.
Mathur VS, Olson JL, Darragh TM, et al. Polyomavirus-induced interstitial nephritis in two renal transplant recipients: case reports and review of the literature. Am J Kidney Dis 1997;29:754–758.
Nickeleit V, Hirsch HH, Binet IF, et al. Polyomavirus infection of renal allograft recipients: from latent infection to manifest disease. J Am Soc Nephrol 1999;10:1080–1089.
Ramos E, Drachenberg CB, Papadimitriou JC, et al. Clinical course of polyoma virus nephropathy in 67 renal transplant patients. J Am Soc Nephrol 2002;13:2145–2151.
Randhawa PS, Finkelstein S, Scantlebury V, et al. Human polyoma virus-associated interstitial nephritis in the allograft kidney. Transplantation 1999;67:103–109.
Josephson MA, Williams JW, Chandraker A, et al. Polyomavirus-associated nephropathy: update on antiviral strategies. Transpl Infect Dis 2006;8:95–101.
Colvin RB . Antibody-mediated renal allograft rejection: diagnosis and pathogenesis. J Am Soc Nephrol 2007;18:1046–1056.
Feucht HE, Schneeberger H, Hillebrand G, et al. Capillary deposition of C4d complement fragment and early renal graft loss. Kidney Int 1993;43:1333–1338.
Herzenberg AM, Gill JS, Djurdjev O, et al. C4d deposition in acute rejection: an independent long-term prognostic factor. J Am Soc Nephrol 2002;13:234–241.
Racusen LC, Colvin RB, Solez K, et al. Antibody-mediated rejection criteria - an addition to the Banff 97 classification of renal allograft rejection. Am J Transplant 2003;3:708–714.
Campbell RD, Gagnon J, Porter RR . Amino acid sequence around the thiol and reactive acyl groups of human complement component C4. Biochem J 1981;199:359–370.
Mold C, Bradt BM, Nemerow GR, et al. Epstein-Barr virus regulates activation and processing of the third component of complement. J Exp Med 1988;168:949–969.
Spiller OB, Morgan BP . Antibody-independent activation of the classical complement pathway by cytomegalovirus-infected fibroblasts. J Infect Dis 1998;178:1597–1603.
Honsova E, Lodererova A, Viklicky O, et al. BK-virus nephropathy and simultaneous C4d positive staining in renal allografts. Cesk Patol 2005;41:163–166.
Ott U, Steiner T, Busch M, et al. A single-center experience with BK virus nephropathy. Clin Nephrol 2008;69:244–250.
Meehan SM, Kadambi PV, Manaligod JR, et al. Polyoma virus infection of renal allografts: relationships of the distribution of viral infection, tubulointerstitial inflammation, and fibrosis suggesting viral interstitial nephritis in untreated disease. Hum Pathol 2005;36:1256–1264.
Bracamonte E, Leca N, Smith KD, et al. Tubular basement membrane immune deposits in association with BK polyomavirus nephropathy. Am J Transplant 2007;7:1552–1560.
Hever A, Nast CC . Polyoma virus nephropathy with simian virus 40 antigen-containing tubular basement membrane immune complex deposition. Hum Pathol 2008;39:73–79.
Racusen LC, Solez K, Colvin RB, et al. The Banff 97 working classification of renal allograft pathology. Kidney Int 1999;55:713–723.
Solez K, Colvin RB, Racusen LC, et al. Banff 07 classification of renal allograft pathology: updates and future directions. Am J Transplant 2008;8:753–760.
Batal I, Girnita A, Zeevi A, et al. Clinical significance of the distribution of C4d deposits in different anatomic compartments of the allograft kidney. Mod Pathol 2008;21:1490–1498.
Kayler LK, Kiss L, Sharma V, et al. Acute renal allograft rejection: diagnostic significance of focal peritubular capillary C4d. Transplantation 2008;85:813–820.
Randhawa P, Ho A, Shapiro R, et al. Correlates of quantitative measurement of BK polyomavirus (BKV) DNA with clinical course of BKV infection in renal transplant patients. J Clin Microbiol 2004;42:1176–1180.
Batal I, Chalasani G, Wu C, et al. Deposition of complement product C4d in anti-glomerular basement membrane glomerulonephritis. Am J Kidney Dis 2009;53:1098–1101.
Celik B, Randhawa PS . Glomerular changes in BK virus nephropathy. Hum Pathol 2004;35:367–370.
Acknowledgements
This work was supported by grants NIH R01-51227 and NIH RO1-06330.
Author information
Authors and Affiliations
Corresponding authors
Additional information
This work was presented at the United States and Canadian Academy of Pathology, Annual Meeting, Boston, 2009.
Disclosure/conflict of interest
The authors declare no conflict of interest.
Rights and permissions
About this article
Cite this article
Batal, I., Zainah, H., Stockhausen, S. et al. The significance of renal C4d staining in patients with BK viruria, viremia, and nephropathy. Mod Pathol 22, 1468–1476 (2009). https://doi.org/10.1038/modpathol.2009.118
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/modpathol.2009.118