Abstract
Human Staphylococcus aureus (SA) nasal carriage provides a reservoir for the dissemination of infectious strains; however, factors regulating the establishment and persistence of nasal colonization are mostly unknown. We measured carriage duration and nasal fluid inflammatory markers after nasally inoculating healthy participants with their previously isolated SA strains. Out of 15 studies, 10 resulted in rapid clearance (9±6 days) that corresponded with upregulated chemokines, growth factors, and predominantly Th1-type cytokines, but not interleukin (IL)-17. Nasal SA persistence corresponded with elevated baseline levels of macrophage inflammatory protein-1β, IL-1β, and IL-6, no induction of inflammatory factors after inoculation, and decreased IL-1 receptor antagonist/IL-1β ratio. SA-expressed staphylococcal protein A (SpA) levels correlated positively with carriage duration. Competitive inoculation studies revealed that isogenic SpA knockout (ΔSpA) strains were cleared faster than wild type only in participants with upregulated inflammatory markers after inoculation. The remaining participants did not mount an inflammatory response and did not clear either strain. ΔSpA strains demonstrated lower growth rates in carrier nasal fluids and lower survival rates when incubated with neutrophils. Collectively, the presented studies identify innate immune effectors that cooperatively modulate nasal carriage duration, and confirm SpA as a bacterial codeterminant of SA nasal carriage.
Similar content being viewed by others
Introduction
Complications from Staphylococcus aureus (SA) skin and soft tissue infections, surgical site infections, and bacteremia remain an important public health concern as antibiotic-resistant strains continue to emerge and healthcare costs continue to rise. Interestingly, SA colonization of humans is nearly always nonpathogenic and most people become colonized with SA transiently throughout their lives. Approximately 30% of healthy adults carry SA asymptomatically at any given moment, regardless of environment, with higher carriage rates and clinical infections in children and those with diabetes, obesity, or certain genetic polymorphisms or drug regimens affecting innate immunity.1, 2, 3, 4, 5, 6 The primary reservoir for SA in humans is the nasal vestibule, and it is now realized that clinical and methicillin-resistant SA strains are nasally carried by the general public.7, 8, 9 As nasal carriers of SA easily transmit their infectious strains and are themselves at risk for extranasal SA infections with their nasally carried strain,10, 11 factors controlling the duration of nasal colonization warrant further investigation.
We have used a combination of in vivo and in vitro approaches to explore specific determinants of human SA nasal carriage. Human autologous nasal inoculation studies revealed that coordinated induction of innate mucosal inflammatory factors associates with nasal SA clearance. We also explored the connection between staphylococcal protein A (SpA) and SA capacity for survival in the human nasal environment, and determined that levels of expressed SpA protein correlate with longer nasal carriage duration, whereas stains lacking SpA protein exhibited decreased survival upon exposure to nasal fluid and neutrophils.
Results
Distinct SA nasal carriage trends among healthy individuals
To investigate early events in nasal SA colonization, we designed a human nasal inoculation protocol using a healthy adult cohort that had been monitored for SA carriage for 1–3 years by our laboratory.8 Participants were cleared of nasal SA through a twice-daily topical application of the antibiotic mupirocin for 5 days. At 1 week after the last application, clearance of SA was confirmed and nasal fluids were collected for baseline (day −7) measurements of nasal mucosal inflammatory factors. One additional week later, participants were inoculated in each nostril with 2 × 107 colony-forming units (CFUs) of donor-matched (autologous) SA that had been isolated and genotyped from a prior study visit. Nasal SA load was monitored twice weekly for 30–35 days, and nasal secretions were collected at 3–4-day intervals for 2 weeks, followed by weekly collections for another 2 weeks.
A total of 15 experimental inoculations were performed on 8 individuals, with 5 subjects (D528, D547, D720, D830, and D831) participating 2–3 times over a 1-year period (Figure 1). Participant and strain information are shown in Table 1. All but one participant was designated as an intermittent nasal SA carrier as repeated samplings demonstrated at least one visit in which SA (CFU/swab) was not detected in either nostril. Participant D720 was considered a persistent carrier based on 9 out of 9 SA-positive nasal swab samples taken during 18 months of monitoring.8 Non-SA nasal bacteria levels decreased expectedly following the topical mupirocin regimen (day −7); however, levels rebounded by inoculation day (day 0), and were steady for the duration of the month-long observation period (Figure 1a). In 10 of the 15 studies, clearance of SA from the nares occurred within 9±6 (mean±s.d.) days, with all participants clearing SA by day 20 (Figure 1b). Among the five studies in which nasal SA was not cleared by the end of the month-long follow-up period (Figure 1c), all exhibited at least a 2-log reduction in SA CFUs during the month. Three of the five participants’ nasal SA decreased below the level of detection at 1–2 visits during days 14–28, although levels rose again by days 31–35 (Figure 1c, participants D547, D720, and D831). Participants D547, D720, D830, and D831 all experienced one inoculation study in which nasal SA persisted to the end of the follow-up period (Figure 1c), whereas clearance occurred in replicate studies utilizing the same autologous SA isolate (Figure 1b). These different outcomes underscore the complex nature of interactions between SA and human nasal mucosa, and suggest that the host response to SA, more than SA strain genotypic attributes, modulates carriage duration. Furthermore, clearance of nasal SA by D720 (D720 inoculation 2 in Figure 1b) indicates that even carriers designated as “persistent” are capable of clearing nasal SA.
Autologous nasal Staphylococcus aureus (SA) inoculation revealed distinct carriage patterns in healthy humans. Nasal SA carriers were treated for 5 days with topical mupirocin to clear SA. At 2 weeks after the last mupirocin application, subjects were intranasally inoculated on two consecutive days with their own previously isolated nasal SA. Left and right nostril swabs were performed before mupirocin application, 1 week after mupirocin application (day −7), at inoculation (days 0–1), and at 2–4-day intervals after inoculation. Commensal and SA colony-forming units (CFUs) enumerated from left and right nostrils were averaged. In total, 15 independent inoculation studies were performed on 8 participants (subjects and inoculation (“Inoc”) numbers denoted to the right of b and c). (a) Non-SA CFUs before and after mupirocin application and at subsequent visit intervals. *P<0.05 vs. before mupirocin application. Error bars indicate mean±s.e.m. (b) SA CFUs for 10 inoculation studies in which nasal SA was cleared by day 20 for all subjects. (c) SA CFUs for 5 studies in which nasal SA was not cleared within 1 month. Dotted horizontal lines in b and c indicate the limit of detection.
The combined upregulation of chemokines, growth factors, and inflammatory cytokines associated with nasal SA clearance
Previous studies showed that nasal secretions from SA carriers support the in vitro growth of SA more than secretions from healthy noncarriers,12, 13 suggesting that host factors play a major role in either the onset or duration of colonization or both. It has also been demonstrated that nasal SA carrier fluids have higher levels of antimicrobial peptides HNP-1 (human neutrophil peptide 1), HNP-3 (human neutrophil peptide 3), and HBD-2 (human β-defensin 2) compared with noncarrier fluids,13, 14 indicating a local innate immune response to colonizing SA that might prevent carriage symptoms even without accomplishing complete SA clearance. In order to determine additional inflammatory modulators that contribute to human nasal host defense against SA in vivo, we performed a 27-plex cytokine/chemokine/growth factor assay on each collected nasal fluid from the 15 autologous inoculation studies, and stratified the data according to whether participants’ nasal SA was cleared (“clearance” group) or not (“carriage” group) during the subsequent month of follow-up. Figure 2 presents the sum of 16 detectable nasal fluid analytes at day −7 (pre-inoculation) and day 2 (after inoculation) for each of the clearance and carriage groups. The total level of expressed host response factors (interleukin (IL)-8, interferon-γ-inducible protein 10 (IP-10), monocyte chemoattractant protein-1 (MCP-1), Eotaxin, macrophage inflammatory protein-1β (MIP-1β), interferon-γ (IFN-γ), IL-6, tumor necrosis factor-α (TNF-α), IL-1β, interleukin-1 receptor antagonist (IL-1RA), granulocyte colony-stimulating factor (G-CSF), vascular endothelial growth factor (VEGF), fibroblast growth factor (FGF), platelet-derived growth factor (PDGF), IL-7, and IL-12 p70) was significantly upregulated after inoculation only during nasal clearance of SA (P=0.0098, Figure 2). Among the 27 proteins measured in the multiplex assay, the following were either undetectable or expressed at very low concentrations (<10 pg per nasal fluid (NF) sample) by all participants at all sampling times: IL-2, IL-4, IL-5, IL-9, IL-10, IL-13, IL-15, IL-17, MIP-1α, RANTES (regulated on activation, normal T cell expressed and secreted), and granulocyte-macrophage colony-stimulating factor (GM-CSF).
Aggregate nasal mucosal response to Staphylococcus aureus (SA) inoculation associates with clearance. Collected nasal fluids from the autologous inoculation studies were analyzed by multi-plex bead array (Bio-Plex). Assay data (pg ml−1) were normalized to the volume of each collected nasal fluid. Cumulative expression levels of all 16 detectable analytes before inoculation (day −7) and after inoculation (day 2) for 15 nasal inoculation (Inoc) studies are presented. Red circles indicate the 10 participants who cleared nasal SA, and green circles represent the 5 participants in whom nasal SA carriage persisted during the month of follow-up. Error bars indicate mean±s.e.m.
Disparate baseline and induced host inflammatory mediator profiles between nasal SA clearance and carriage groups
Baseline (day −7) levels of the CC chemokine MIP-1β (CCL4) and the inflammation markers IL-1β and IL-6 were significantly higher in the SA carriage group compared with the clearance group (Figure 3). In the clearance group only, levels of IL-6 and IL-8 were elevated at all postinoculation time points compared with baseline. Similarly, IL-1β was not significantly upregulated after inoculation in participants who carried SA but was elevated at postinoculation days 2 and 7, and again at week 4 in the clearance group. IL-1β activity is counteracted at mucosal surfaces by high levels of IL-1RA. Levels of IL-1RA in nasal fluid were similar between the clearance and carriage groups (averaging 100 pg per collected NF (ng ml−1 levels) for most participants); however, only the clearance group exhibited induced nasal IL-1RA after SA inoculation (days 7, 10, and 23–30; Figure 3). Nasal fluid G-CSF was elevated after SA in the clearance group and in the majority of participants in the carriage group, supporting the notion that neutrophil recruitment to the nasal mucosa keys the human innate nasal response to SA exposure. The remaining detectable nasal fluid analytes are displayed in Supplementary Figure S1 online. Notably, IL-7 and IL-12 p70 (T-cell stimulation/maturation factors), IP-10 (CXC chemokine CXCL10), TNF-α, IFN-γ, FGF, and PDGF were induced at most postinoculation time points in the clearance group but not in individuals experiencing prolonged carriage. Collectively, Figures 2 and 3 suggest that a coordinated acute inflammatory response in the nasal mucosa shortens the duration of SA carriage.
Induction of proinflammatory cytokines, chemokines, and growth factors correlates with nasal Staphylococcus aureus (SA) clearance. Healthy participants were nasally inoculated with autologous SA as described in Figure 1, and nasal fluids were collected at the indicated days before (day −7) or after inoculation and analyzed by multi-plex bead array as described in Figure 2. Red and green circles indicate clearance and carriage groups, respectively. **Baseline differences between the clearance and carriage groups, with P values displayed in the graphs. *P<0.05 vs. baseline (day −7) for the clearance group. #P<0.05 vs. baseline (day −7) for the carriage group. Error bars indicate mean±s.e.m. IL, interleukin; IL-1RA, interleukin-1 receptor antagonist; G-CSF, granulocyte colony-stimulating factor; MIP-1β, macrophage inflammatory protein-1β; NF, nasal fluid.
Low IL-1RA/IL-1β ratio is associated with nasal SA persistence
We next calculated the ratio of IL-1RA to IL-1β for each collected nasal fluid, and compared the clearance and carriage groups. Participants who successfully cleared nasal SA exhibited a mean IL-1RA/IL-1β ratio of ≥100 at baseline and at day 7 and onward after SA inoculation (Figure 4), with the decreased ratio at day 2 corresponding with the observed induction of IL-1β (Figure 3) immediately after SA inoculation. Participants who failed to clear nasal SA presented a mean nasal fluid IL-1RA/IL-1β ratio ranging from 10 to 30 at all days except day 16 (ratio ∼80, Figure 4), correlating with a decrease in mean expression of IL-1β at that time point (Figure 3). The nasal fluid IL-1RA/IL-1β ratio may therefore represent a metric for anti-SA function in the nasal mucosa, with the observed differences between the clearance and carriage groups indicating that dysregulated IL-1β-mediated signaling, or the introduction of non-SA subclinical nasal stimuli capable of augmenting IL-1β levels, may contribute to SA persistence.
Decreased IL-1RA/IL-1β ratio in the nasal mucosa of participants who failed to clear nasal Staphylococcus aureus (SA). The IL-1RA/IL-1β ratio was calculated for each nasal fluid that was collected on the indicated days during the nasal inoculation studies. *Differences between the clearance (red circles) and carriage (green circles) groups, with P values displayed in the graph. Error bars indicate mean±s.e.m. IL-1β, interleukin-1β; IL-1RA, interleukin-1 receptor antagonist.
SA SpA levels correlate with human nasal carriage duration
Previous proteomic analyses of SA nasal carriage strains revealed a potential correlation between levels of SA-expressed SpA and carriage persistence.15 For each of the strains used in the autologous inoculation experiments presented above, we obtained the spa type, spa polymorphic X region short sequence repeat number, and SpA protein level (Table 1). Mean SpA protein levels correlated positively with nasal carriage duration observed in the 15 nasal inoculation studies (Figure 5a; r=0.45, P=0.046), whereas there was no correlation between strains’ spa short sequence repeat number and carriage duration (Figure 5b; r=0.03, P=0.522). We next evaluated whether SpA protein level correlated with induction of inflammatory markers observed 2 days after inoculation compared with baseline. Fold induction values calculated for each of the 16 detectable analytes were averaged and plotted against the mean SpA protein value for the corresponding SA. There was no correlation between SpA protein level and the average induction of inflammatory markers (Figure 5c; r=0.0371, P=0.4478), nor was there a correlation between SpA level and the fold induction of any individual inflammatory marker (data not shown). As SpA has been demonstrated by other groups to promote activation of epithelial cells through TNF receptor 1 signaling and nuclear factor (NF-κB) activation,16, 17, 18 we similarly assessed the effect of each SA strain on human nasal epithelial cell (NEC) NF-κB activity in vitro. When NECs were grown at the air liquid interface and topically applied with SA at a multiplicity of infection of 5, there was indeed a strong correlation between SpA level and phosphorylation of the NF-κB subunit p65 (Figure 5d; r=0.84, P=0.002). Conversely, the amount of SpA in individual strains did not affect the invasion of NECs in vitro (Figure 5e; r=0.29, P=0.483). Strains 2098 and 2024 were not included in the analysis of the in vivo inoculation experiments because of missed visits and incomplete carriage data, but were of interest because of their vastly different SpA levels (Table 1). Notably, they also induced NEC NF-κB activity proportionally to their SpA levels (27.3±1.6-fold induction of NF-κB by 2098 and 8.8±0.5-fold by 2024, data not shown). Taken together, these studies provide human in vivo evidence that elevated expression of SpA at the protein level supports SA nasal colonization. As a failed acute inflammatory response to nasal SA also correlated with carriage (Figures 2 and 3), the in vitro assays indicating a role for SpA in activating epithelial NF-κB activity may not adequately model the asymptomatic (subclinical) SA–host interactions that occur in human nasal mucosa.
Staphylococcus aureus (SA) staphylococcal protein A (SpA) levels correlate with nasal carriage duration and nasal epithelial cell nuclear factor (NF)-κB activity. SA strains were prepared as for the nasal inoculations and then assayed for SpA content by enzyme-linked immunosorbent assay (ELISA). (a) Mean SpA values (measured from three individual frozen stocks of each strain, shown in Table 1) correlated positively with the number of days that SA strains survived in their host during the autologous inoculation studies. (b) spa polymorphic X region short sequence repeat (SSR) number did not correlate with carriage duration. (c) SpA level did not correlate with the average fold induction of 16 inflammatory markers 2 days after inoculation in vivo. SpA protein level correlated strongly with the in vitro induction of NF-κB activity in cultured nasal epithelial cells (NECs) (d), but not with the ability to invade NECs in vitro (e). Correlation coefficients (Pearson’s r), P values, and linear regression lines are shown in each graph. CFU, colony-forming unit.
SA mutants lacking SpA were cleared faster than wild-type counterparts during competitive nasal inoculation
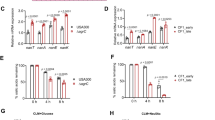
We next pursued direct human in vivo evidence that SpA is an important bacterial determinant of nasal carriage. In a subset of participant strains in our cohort, representing diverse spa types and SpA expression levels, we performed site-directed spa gene disruption using the TargeTron gene knockout system.19 An advantage of TargeTron methodology is the lack of antibiotic-selectable markers, thus eliminating the possibility of introducing antibiotic-resistant SA strains during nasal inoculation. The absence of SpA protein expression (ΔSpA) in participant strains 547, 830, 2024, and 2098 was confirmed by western blot (Figure 6a) and commercial enzyme-linked immunosorbent assay (ELISA; not shown). Strain fitness was assessed by performing growth assays in nutrient-rich tryptic soy broth (Figure 6b), low serum cell culture media containing fewer nutrients (Figure 6c), and on confluent NEC layers grown at the air liquid interface (Figure 6d): each assay demonstrated no growth differences between the wild-type (WT) and ΔSpA strains. We also measured NEC-associated vs. extracellular SA after 2 h of incubation and observed that WT and ΔSpA strains were similarly able to attach/invade nasal epithelium, with 2024 ΔSpA demonstrating more cell association than its counterpart WT strain (Figure 6e).
Knockout of staphylococcal protein A (SpA) does not alter growth kinetics or adhesion to nasal epithelial cells (NECs). (a) Western blot analysis of SpA expression in wild-type (WT) and SpA-knockout (ΔSpA) strains, compared with recombinant SpA (control, left lane). Growth kinetics of WT (solid lines) and ΔSpA (dotted lines) strains in nutrient-rich media (b), lower nutrient cell culture media (c), and on the surface of NECs (d), with color scheme for b–d indicated; n=3–4 for each strain in each assay. (e) WT and ΔSpA strains were applied to NECs (multiplicity of infection (MOI) 5) for 2 h, then evaluated for adhesion/entry; n=4 for each strain. (b–e) Error bars indicate mean±s.e.m. CFU, colony-forming unit; DMEM, Dulbecco’s modified Eagle’s medium; FBS, fetal bovine serum.
Next, we performed competitive nasal inoculation studies using equivalent amounts of WT and ΔSpA autologous SA in each nostril, monitored nasal SA load, and collected nasal fluids for the assessment of host inflammatory responses. Upon analyzing the average fold change in inflammatory factor levels (same 16 analytes listed above in the description of Figure 2) for 7 independent inoculation experiments, the experiments were observed to naturally stratify by the demonstrated nasal mucosal host response: 4 inoculations elicited increased nasal fluid inflammatory factors, whereas 3 inoculations resulted in decreased levels compared with preinoculation (Figure 7a). In the former group, ΔSpA strains were cleared significantly faster than WT (Figure 7b; log rank: χ2=4.051, P=0.044). The latter group resulted in carriage of both WT and ΔSpA strains at similar levels throughout the month-long follow-up (Figure 7c; log rank: χ2=0.004, P=0.948).
Participants mounting a mucosal response to inoculated Staphylococcus aureus (SA) cleared ΔSpA variants faster than wild-type (WT) strains. SA mutants lacking staphylococcal protein A (SpA) protein (ΔSpA) were created for strains 2024, 547, 2098, and 830. Seven competitive inoculation experiments were performed by intranasally administering equivalent amounts of WT and ΔSpA SA to each nostril of the donor-matched (autologous) human host. Individual experiments (participant number and inoculation experiment (“Expt”) number) are indicated in a. Collected nasal fluids were analyzed by multi-plex bead array and the fold change for each of the 16 detectable analytes, relative to baseline, were averaged and plotted for each participant at the indicated time points (a). Dotted line marks the threshold between elevated (>1-fold) and decreased (<1-fold) analyte levels compared with baseline. Based on the corresponding nasal SA colony-forming unit (CFU) values, survival curves (WT vs. ΔSpA) were generated for four studies (green, orange, pink, and purple in a) that demonstrated a positive inflammatory response to SA inoculation (b) and the three studies (red, black, and blue in a) exhibiting no induction of inflammatory mediators in response to SA inoculation (c).
Nasal SA strains lacking SpA exhibited reduced survival in nasal fluid and during neutrophil exposure
We next explored whether ΔSpA strains were less likely to persist in an environment of human nasal fluid. To develop a suitable assay, five natural SA strains from our cohort were grown to log phase as in the inoculation studies, then washed and incubated with various nutrient buffers or heat-inactivated nasal fluid (thus no endogenous CFUs) for 24 h (Figure 8a). With an input of 50,000–100,000 CFUs, no live SA was recovered when strains were incubated with nasal fluid stimulant buffer (NFB) containing electrolytes and supplemented with 6 g l−1 glucose, whereas provision of 0.2% serum (a source of iron and hemoglobin known to promote SA nasal colonization20, 21) permitted a modest glucose dose dependence, yielding 1.5-3-fold growth with 2–6 g l−1 glucose supplementation (Figure 8a). Incubation of SA strains in noncarrier nasal fluid and SA carrier fluid promoted 100–300-fold growth, with carrier fluid providing greater growth potential (Figure 8a; P=0.013, n=5). SA growth in Dulbecco’s modified Eagle’s medium (DMEM) cell culture media (containing salts, amino acids, vitamins, pyruvate, and 4.5 g l−1 glucose) was dependent on serum supplementation: 100-fold growth in 0.2% serum and nearly 1,000-fold growth in 2% serum. Next, growth of WT vs. ΔSpA strains was tested when suspended in DMEM and 2% fetal bovine serum (FBS; Figure 8b) or plain NFB (Figure 8c) and then admixed with eight volumes of SA carrier nasal fluid. When nutrients were higher, all WT and ΔSpA strains propagated similarly (Figure 8b); however, when incubated in the NFB/nasal fluid blend, the ΔSpA variants of strains 547, 2024, and 2098 exhibited significantly decreased growth compared with their WT counterparts (Figure 8c).
Attenuated growth of ΔSpA vs. wild-type (WT) Staphylococcus aureus (SA) in nasal fluid. (a) Growth of representative SA strains from our cohort (2024, 528-11, 713-4, 757-5, 720-7) was assessed in the presence of nasal fluid buffer (NFB) with various indicated additives, non-SA carrier nasal fluid (NCF), SA carrier nasal fluid (CF), or Dulbecco’s modified Eagle’s medium (DMEM) culture media containing 0.2–2% fetal bovine serum (FBS). (b) SA suspended in nutrient-rich DMEM/2% FBS and then mixed with nasal fluid. (c) SA suspended in glucose-free/serum-free nasal fluid buffer and then mixed with nasal fluid. (a–c) N=4–5 for all assay conditions. Error bars indicate mean±s.e.m. SpA, staphylococcal protein A.
As nasal SA clearance has been demonstrated to be dependent on neutrophil recruitment and phagocytosis,22 we also tested whether WT and ΔSpA strains differed in their ability to survive neutrophil exposure. Strains were incubated alone or with primary human neutrophils (multiplicity of infection of 1, n=4 neutrophil preparations from separate donors) for 1 or 3 h. Surviving CFUs were enumerated and percent survival was calculated for each strain. Figure 9 demonstrates a significant trend that ΔSpA strains were more vulnerable to neutrophils compared with WT. Collectively, the results shown in Figures 7, 8, 9 indicate that mucosal host defense regulation is an important determinant of SA nasal carriage duration while SpA levels play a role in codetermining carriage.
Decreased ΔSpA Staphylococcus aureus (SA) survival upon exposure to primary neutrophils. Wild-type (WT) and ΔSpA SA strains were incubated alone or in the presence of primary neutrophils, and % survival was calculated relative to the neutrophil-free condition for each strain. Data represent four independent experiments, each of which used a unique donor neutrophil preparation. Dotted line at 100% represents the SA input. Error bars indicate mean±s.e.m. *Statistical significance between WT and ΔSpA SA strains, with the P values shown in the graph. PMN, polymorphonuclear leukocyte; SpA, staphylococcal protein A.
Discussion
Human nasal carriage of SA is established because of a complex interplay between bacterial virulence factors, host surveillance and defense mechanisms, and nutrient availability. Numerous small animal models of SA infection have enabled the discovery of bacterial factors influencing nasal colonization events including clumping factor B,23 wall teichoic acid,24, 25 and iron-regulated surface proteins IsdA and IsdH.21 On the host side, younger age, obesity, male gender, tobacco use, vitamin D levels, and certain genetic polymorphisms have been implicated as risk factors for nasal SA carriage,26, 27 although the underlying mechanisms remain elusive. Clinical studies have demonstrated that nasal carriage isolates match the invasive strain in the majority of cases,10, 11, 28, 29 and that screening and decolonization procedures associate with decreased extranasal infections, mortality, and medical costs.30, 31 As the emergence of antibiotic-resistant SA is of critical concern worldwide, new protocols for studying early colonization events, immune tolerance, and clearance mechanisms in human nasal SA carriage will be essential for development of advanced therapeutics.
We evaluated the role of innate nasal host defense against SA by performing autologous nasal inoculations of known SA carriers. Participants reported no deleterious effects, and by using this approach we revealed in vivo that the nasal carriage state is influenced by the host’s early inflammatory response to SA colonization. Figures 2 and 3 indicate that clearance of SA from the human nasal mucosa correlates with a coordinated induction of inflammatory mediators IL-1β, IL-6, TNF-α, and IFN-γ; chemokines IL-8, MIP-1β, and IP-10; and growth factors/T-cell maturation factors VEGF, FGF, PDGF, IL-12 p70, and IL-7. In the majority of experiments, hosts mounted this robust defense by day 2 that corresponded with rapid SA nasal clearance. SA persistence occurred in only 5 of 15 experiments, and corresponded with the host failing to elaborate an induction of these factors following SA inoculation. Surprisingly, IL-17, previously shown to mediate SA clearance in mice,22 was not detected in nasal secretions. It is possible that IL-17 could be cell associated or insoluble in nasal secretions, or that sub-pg ml−1 levels of IL-17 expression is enough to activate local T lymphocytes. Nevertheless, the observed Th1-type dominant cytokine response is not surprising on account of its known association with epithelia-derived and neutrophil-mediated immunity. In contrast, the observed 10/15 clearance rate was unexpected, as we initially surmised that upon reintroducing a participant’s own SA isolate, nasal carriage would be easily restored. As identical SA isolates could persist in one inoculation experiment but not in the next, or vice versa, in the same participant (Figure 1), the collective data suggest that the carriage state is predominantly defined by the host’s varied ability to respond to SA.
Although all participants in the 15 nasal inoculation experiments were healthy and asymptomatic, baseline nasal fluid cytokine and chemokine levels were generally higher for the individuals who failed to clear SA than for the clearance group (Figures 2 and 3), whereas the IL-1RA/IL-1β ratio was consistently lower in the carriage group (Figure 4). It is not currently understood how or why elevated baseline levels of MIP-1β, IL-1β, and IL-6 would not prime the host for an effective anti-SA response. It is possible that a non-SA inflammatory stimulator encountered the nasal mucosa during the postmupirocin phase and contributed to the failed induction of IL-1β and other inflammatory mediators after SA inoculation by shifting the host response toward alternate signaling pathways. Such a scenario, marked by a low IL-1RA/IL-1β ratio, may have provided SA with the necessary “window of opportunity” for successful colonization of the nasal epithelial barrier.
We enumerated the non-SA nasal microbes but did not identify all of the represented species. Notably, we did check for the presence of Esp-secreting Staphylococcus epidermidis (SE), reported to inhibit SA biofilm formation and nasal colonization,32 in 8 participants (11 studies, data not shown). Of the SE isolates, 83% (103 of 124 colonies) were esp positive, suggesting that this species is common in our cohort of nasal SA carriers. Three participants were cocolonized with SA and esp-positive SE beyond 25 days, whereas 2 participants with the fewest number of esp-positive SE swabs (5 out of 12 and 3 out of 11, respectively) cleared SA within 1 week. Therefore, it cannot be concluded from this subset of individuals that SA clearance correlated with Esp-secreting SE load. Regardless, the issue of competition between nasal SA and endogenous flora will be an important consideration going forward. It was recently observed that increased SA clearance occurred after skin-to-skin contact between noncarrier mothers and nasal SA-positive newborns,33 and microbiota-induced neutrophil recruitment and T-cell maturation correlated with defense against SA in mice.34 Commensal–host cell interaction has additionally been demonstrated to dramatically affect skin immunity.35 These important studies support the idea that a diverse and dynamic nasal microflora may hamper SA colonization, and thus further study of endogenous nasal flora during natural transitioning in and out of SA carriage is needed.
Numerous studies using mouse models or in vitro cell culture assays have demonstrated the importance of SpA as a SA virulence factor mediating TNF receptor 1-induced inflammatory cascades, opsonophagocytosis, and B-cell apoptosis.16, 17, 18, 36 We and others previously showed a relationship between SpA expression levels,15, 37 but not spa polymorphisms,38 and human nasal carriage persistence. In the present in vivo studies there was a correlation between SpA protein level, but not SpA short sequence repeat number and nasal carriage duration (Figure 5). During competitive inoculation studies using equivalent amounts of WT and ΔSpA autologous SA, ΔSpA variants were eliminated from the nose earlier than WT only when the host mounted a coordinated inflammatory defense (Figure 7). Taken together, these findings suggest that the predominant nasal mucosal response to asymptomatic SA involves innate immune effectors culminating in neutrophil-mediated clearance, rather than antibody-mediated defenses that play a larger role during clinical infection. We have not characterized the anti-SA antibody profiles of carriers in our cohort, and thus cannot speculate about the role of seropositivity in the clearance of WT and ΔSpA strains or the preferential clearance of ΔSpA. However, essentially all people, regardless of carriage status, make immunoglobulins against SA antigens, and experimental nasal inoculation did not alter anti-SA antibody profiles or humoral responses in healthy participants in a previous report.39, 40, 41 Persistent nasal carriers have a greater risk of clinical SA infection with their endogenous strain, but make more SA-neutralizing antibodies than noncarriers and have a lower risk of death from SA bacteremia.10, 40 Together, these studies imply that anti-SA antibody status is the result of extranasal SA exposure, and not a modulator of nasal carriage status or duration. Similarly, the enhanced susceptibility of ΔSpA strains to nasal fluid and neutrophils (Figures 8 and 9), and the known role of SpA in epithelial TNF receptor 1 shedding,17 suggest that the role of SpA in modulating nasal carriage duration is linked to influences on innate rather than adaptive host defenses.
In summary, our findings demonstrate the extent to which innate immune effectors of the nasal mucosa influence the duration of asymptomatic human SA carriage. In light of recent evidence suggesting that noncarriers are actually very rare, and that most of the population is colonized transiently,4, 42 the investigation of clearance mechanisms in SA nasal carriers is urgently needed. Current vaccine design efforts show promise for protecting the host from SA deep tissue infections and bacteremia,43, 44 but it is likely that development of improved nasal decolonization strategies will involve a distinct set of molecular targets.
Methods
Participants, informed consent, and ethics
Nine healthy SA nasal carriers (5 males and 4 females, range 22–47 years old) were selected from a cohort of 109 healthy individuals whose carriage status was monitored longitudinally for up to 3 years. Studies were performed with approval of the institutional review board of University of Central Florida, fully accredited by the Association for the Accreditation of Human Research Protection Programs. Participants provided consent at each study visit, and there were no adverse effects of nasal SA reported during the study.
Isolation, storage, and use of nasal SA isolates
To isolate nasal SA, the anterior region of each nostril was swabbed with sterile polyester-tipped swabs, and each swab was swirled in 2 ml of TSB (Bacto tryptic soy broth, Becton Dickinson (BD), Franklin Lakes, NJ) to extract microbes and prepare an initial glycerol stock (overnight incubation at 37°C/250 r.p.m., then stored at −80°C supplemented with 15% glycerol). Then, 0.1 ml was plated on tryptic soy agar/5% sheep’s blood (TSA II from BD, hereafter termed “blood agar”) and incubated for ∼18 h at 37°C then colonies were identified as SA using the Staphyloslide Latex Test reagent (BD). SA colonies were propagated in TSB and then (i) genotyped by multilocus sequence typing and spa typing using primers and PCR conditions described previously,8, 45, 46 (ii) stored as colony glycerol stocks at −80°C and (iii) prepared as ready-to-use glycerol-free stocks by dispensing overnight liquid culture into microtubes and rapidly freezing the aliquots in liquid nitrogen before storage at −80°C. For experiments, stocks were thawed and 1% (v/v) inocula were grown in TSB at 37°C/250 r.p.m. for 2.5 h to achieve log phase growth. SA was collected by centrifugation, washed with Hank’s buffered salt solution (Corning/Cellgro, Tewksbury, MA), and then resuspended in Hank’s buffered salt solution and used for nasal inoculation or treating NECs. At each use, a portion of the SA preparation was plated on blood agar to confirm the inoculum. For the assessment of natural SpA levels of each nasal strain, SA from three representative frozen stocks was handled in the same way as for nasal inoculations, except that Hank’s buffered salt solution–washed bacteria were further processed for SpA ELISA (details below). Proteins were extracted by adding 10 volumes of 10% acetic acid, then vortexed for 30 min. Soluble extracts were clarified by centrifugation, vacuum-dried (SPD1010 SpeedVac, ThermoFisher, Waltham, MA), neutralized to pH 7, and added to the ELISA workflow. For western blot assays (described below), overnight cultures of WT and ΔSpA SA were fractionated as previously described47, 48 for assessing cell wall–associated and secreted levels of SpA.
Autologous nasal SA inoculation and specimen collection
As most participants were nasal carriers who usually tested positive for SA, all participants underwent the same decolonization protocol before initiating the SA inoculation studies. Participants self-administered mupirocin nasal ointment (Bactroban, GlaxoSmithLine, Philadelphia, PA) to each nostril, twice daily for 5 days. At 1 week after the last mupirocin application, nasal swabs were collected to confirm that endogenous SA had been cleared, and to enumerate non-SA CFUs. Nasal fluid was collected by placing a flexible 8-French catheter into the nasal vestibule under low vacuum.13 Individuals typically collected 0.1–1 ml nasal fluid in this manner. Fluids were transferred to microcentrifuge tubes and stored at −80°C until use. These collections were considered to take place on “day −7”. One additional week later, participants’ previously isolated strains were prepared as described above and each nostril was inoculated with 2 × 107 CFUs of SA on two consecutive days (days 0 and 1, respectively). On day 2, and every 2–4 days thereafter for ∼35 days, nasal swabs were performed on each nostril. Nasal fluid was collected at days 2, 7, and 10, then every 4–7 days thereafter. All SA strains were confirmed for mupirocin sensitivity before experimental inoculation; and a subset of enumerated colonies collected during the studies were confirmed by multilocus sequence typing and spa typing8, 45, 46 to match the inoculated strain.
Nasal fluid processing and immunodetection assays
Nasal fluids were thawed on wet ice and vortexed and then pulse-sonicated 30 × 1 s on wet ice at power level 2 (Fisher Scientific Sonic Dismembrator model 100, Pittsburgh, PA) and clarified by centrifugation (13,000 r.p.m. for 5 min at 4°C). Bio-Rad Pro Human Cytokine Assay (Bio-Rad Laboratories, Hercules, CA) was performed for detection of IL-1β, IL-1RA, IL-2, IL-4, IL-5, IL-6, IL-7, IL-8, IL-9, IL-10, IFN-γ, IL-12 (p70), IL-13, IL-15, IL-17, IP-10, MCP-1, MIP-1α, MIP-1β, PDGF-BB, RANTES, TNF-α, Eotaxin, FGF basic, G-CSF, GM-CSF, and VEGF. Standard curves were generated by diluting company-provided recombinant standards in each of three buffer preparations to determine whether the total protein content of standard diluent altered assay sensitivity: DMEM/10% FBS to represent a standard cell culture supernatant, keratinocyte serum-free base medium (Life Technologies, Grand Island, NY) to represent a low protein buffer, and NFB12 (85 mM Na, 97 mM Cl, 20 mM K, 1 mM Ca, 10 mM P, 0.5 mM Mg, and 0.5 mM S) containing 6 mg ml−1 bovine serum albumin as we determined nasal fluids to have an average of 6 mg ml−1 protein. All standard diluents allowed the same level of sensitivity for each analyte. We set a cutoff of 10 pg ml−1 as the detection limit as each standard curve demonstrated good fit down to 7–10 pg ml−1. Company instructions were followed for incubation times, instrument settings (Bio-Rad 200), and BioPlex Pro II wash station settings. Data (pg ml−1) were normalized to the volume of nasal fluid collected and presented as pg per NF sample, as fluid collections >1 ml demonstrated a lower concentration of total protein than typical fluid collections of 0.1–0.5 ml. Normalization of the pg ml−1 data to total protein could not be performed for all of the collected nasal fluids because of volume limitations for certain individuals or collection times.
Human NEC culture, invasion assays, and NF-κB assays
Human NEC (nasal epithelial cells “RPMI 2650” from American Type Culture Collection (ATCC), Manassas, VA) were maintained on 100 mm tissue culture dishes, submerged in 10 ml/dish DMEM high glucose supplemented with 10% FBS, 1 × penicillin–streptomycin solution, and incubated at 37°C/5% CO2. For experiments, cells were transferred to the apical compartment of collagen-coated Transwell inserts (0.4 μm pores, Corning, Corning, NY) such that the cells from each confluent 100 mm dish were seeded on twelve 12 mm or six 24 mm Transwell inserts. After 2 days, basal medium was changed and apical medium was replaced. On day 3, the apical medium was removed and basal medium was changed daily thereafter. On day 6, antibiotics were removed from the medium. Cells were treated with SA on day 9 or 10, when all Transwell cultures demonstrated the ability to seal the basal medium away from the apical chamber, creating an air liquid interface. For SA invasion assays, each strain was topically applied at a multiplicity of infection of 5 that was confirmed by plating an aliquot on blood agar immediately after application to the cells. After 2 h, extracellular bacteria were rinsed off the nasal epithelia and plated on blood agar in serial dilutions. Cell-associated bacteria were enumerated by scraping cells off the Transwells, pulse sonicating on ice (10 × 1 s, power level 4) to disrupt the epithelial cells without harming SA, and plating on blood agar for CFU enumeration the next day. For NF-κB activation assays, treated NECs were rinsed and then lysed and processed for Bio-Plex Pro Cell Signaling assays using Pro Cell Signaling Reagent kit of Bio-Rad. Protein content of each lysate was measured by micro BCA assay (Thermo Scientific Pierce, Pittsburgh, PA), and 20 μg was used for multiplex analysis of phosphorylated-p65 (Ser536) and glyceraldehyde 3-phosphate dehydrogenase (for normalization of NEC content in each sample).
Targeted disruption of SpA in SA nasal carrier strains
Site-directed disruption of the SpA gene (spa) was performed using TargeTron-based insertion of mobile group II introns into spa (Sigma-Aldrich, St Louis, MO), as per the SA-specific procedure described by Yao.19 Because naturally colonizing nasal SA strains were refractory to genetic transformations due to type I and type IV restriction barriers, we modified the TargeTron methodology. The spa-pNL9164 intron insertion plasmids were passaged through high-efficiency Escherichia coli cloning strain DC10B49 before electroporation into SA. Intron insertion was confirmed by PCR and sequence analysis. Loss of SpA protein was confirmed by western blot of SA cell wall and exoproteome fractions (monoclonal antibody ab49734-200, Abcam, Cambridge, MA) and also by ELISA (protein A EIA kit from Enzo Life Sciences, Plymouth, PA). Four different spa-disrupted (ΔSpA) SA nasal strains were generated. Supplementary Table S1 lists all plasmids and oligonucleotides. Growth assays to confirm that ΔSpA strains were not compromised during subcloning and selection were carried out in both liquid culture and on nasal cells as detailed above.
SA CFU microassays with nasal fluids or various nutrient mixtures
SA stocks were thawed and incubated as described above to achieve log phase growth. Bacteria were washed and resuspended in NFB such that OD625=0.2; then diluted 1:100 with either NFB, NFB supplemented with varying amounts of glucose and 0.2% FBS, or DMEM supplemented with 0.2–2% FBS. These diluents or nasal fluids, sonicated as described above and then incubated at 55 °C for 20 min to prevent the growth of endogenous bacteria, were then premixed with SA in microtubes (12 μl NF or diluent +1.5 μl 1:100 SA); then 5 μl per well was plated in duplicate wells of 72-well Terisaki plates (Thermo Scientific Nunc, Waltham, MA) and overlayed with 3 μl per well Chill-out liquid wax (Bio-Rad) to prevent evaporation. One well was immediately plated on blood agar (time 0), and the remaining samples were incubated at 37 °C for 24 h. Sample wells were collected and rinsed, and serial dilutions were plated on blood agar. CFUs were enumerated, and 24 h counts were divided by time 0 counts to obtain fold growth values. Incubations of nasal fluid alone were performed as control to verify that the heating step eliminated the growth of endogenous bacteria. Each SA strain was tested against 3–5 individual nasal fluids, or with the different nutrient mixtures in 3–5 independent assays.
Neutrophil assays
Neutrophils were obtained from freshly drawn whole blood from healthy volunteers50, 51 and incubated overnight at 106 per ml in RPMI-1640 supplemented with 10% FBS using 12-well dishes coated with 1 mg per well poly-L-lysine (Sigma P-9404). The next day, neutrophil media were changed to RPMI/0.5% FBS (R0.5). Log phase SA was washed and diluted 100-fold in R0.5, then 0.1 ml was added to neutrophils in order to achieve the multiplicity of infection of 1 (confirmed by plating on blood agar). Plates were incubated at 37°C/5% CO2 for 1 or 3 h, then cell-free SA was removed from each well, and cell-bound/phagocytosed SA was collected by scraping the cells in a solution of 0.05% Triton X-100 in phosphate-buffered saline (applied 2 min before scraping). All collections were immediately mixed and diluted in R0.5 and plated on blood agar for CFU enumeration. Parallel wells containing SA but no neutrophils were used to control for SA growth, and duplicate wells were used to confirm that Triton treatment did not affect SA CFU counts. Percent survival was calculated for neutrophil-exposed SA relative to cell-free SA ((SAPMN/SAalone) × 100) for each strain. Assays were performed using four separate neutrophil donations.
Statistical analysis
Data were analyzed using GraphPad Prism 5 software (GraphPad Software, La Jolla, CA). For multiplex cytokine data obtained from nasal secretions, total pg of each analyte was calculated based on the volume of nasal fluid collected. For comparing analyte levels or SA survival between groups, paired t-test, unpaired nonparametric Mann–Whitney test, or Wilcoxon rank test were performed according to the data distribution. For the competitive WT/ΔSpA inoculation studies, SA carriage patterns were displayed with Kaplan–Meier survival curves and compared using log-rank (Mantel–Cox) test and median survival time calculations.
References
Kluytmans, J., van Belkum, A. & Verbrugh, H. Nasal carriage of Staphylococcus aureus: epidemiology, underlying mechanisms, and associated risks. Clin. Microbiol. Rev. 10, 505–520 (1997).
van Cleef, B.A., van Rijen, M., Ferket, M. & Kluytmans, J.A. Self-sampling is appropriate for detection of Staphylococcus aureus: a validation study. Antimicrob. Resist. Infect. Control. 1, 34 (2012).
Wertheim, H.F. et al. The role of nasal carriage in Staphylococcus aureus infections. Lancet Infect. Dis. 5, 751–762 (2005).
Miller, R.R. et al. Dynamics of acquisition and loss of carriage of Staphylococcus aureus strains in the community: the effect of clonal complex. J. Infect. 68, 426–439 (2014).
Olsen, K. et al. Obesity and Staphylococcus aureus nasal colonization among women and men in a general population. PLoS One 8, e63716 (2013).
Ruiz, J.N. et al. Nasal vestibulitis due to targeted therapies in cancer patients. Support Care Cancer 23, 2391–2398 (2015).
Lamers, R.P., Stinnett, J.W., Muthukrishnan, G., Parkinson, C.L. & Cole, A.M. Evolutionary analyses of Staphylococcus aureus identify genetic relationships between nasal carriage and clinical isolates. PLoS One 6, e16426 (2011).
Muthukrishnan, G. et al. Longitudinal genetic analyses of Staphylococcus aureus nasal carriage dynamics in a diverse population. BMC Infect. Dis. 13, 221 (2013).
Chen, C.J., Wang, S.C., Chang, H.Y. & Huang, Y.C. Longitudinal analysis of methicillin-resistant and methicillin-susceptible Staphylococcus aureus carriage in healthy adolescents. J. Clin. Microbiol. 51, 2508–2514 (2013).
Wertheim, H.F. et al. Risk and outcome of nosocomial Staphylococcus aureus bacteraemia in nasal carriers versus non-carriers. Lancet 364, 703–705 (2004).
Perl, T.M. et al. Intranasal mupirocin to prevent postoperative Staphylococcus aureus infections. N. Engl. J. Med. 346, 1871–1877 (2002).
Cole, A.M., Dewan, P. & Ganz, T. Innate antimicrobial activity of nasal secretions. Infect. Immun. 67, 3267–3275 (1999).
Cole, A.M. et al. Determinants of Staphylococcus aureus nasal carriage. Clin. Diagn. Lab. Immunol. 8, 1064–1069 (2001).
van Belkum, A. et al. The role of human innate immune factors in nasal colonization by Staphylococcus aureus. Microbes Infect. 9, 1471–1477 (2007).
Muthukrishnan, G. et al. Exoproteome of Staphylococcus aureus reveals putative determinants of nasal carriage. J. Proteome Res. 10, 2064–2078 (2011).
Garofalo, A. et al. The length of the Staphylococcus aureus protein A polymorphic region regulates inflammation: impact on acute and chronic infection. J. Infect. Dis. 206, 81–90 (2012).
Gomez, M.I., O'Seaghdha, M., Magargee, M., Foster, T.J. & Prince, A.S. Staphylococcus aureus protein A activates TNFR1 signaling through conserved IgG binding domains. J. Biol. Chem. 281, 20190–20196 (2006).
Claro, T., Widaa, A., McDonnell, C., Foster, T.J., O'Brien, F.J. & Kerrigan, S.W. Staphylococcus aureus protein A binding to osteoblast tumour necrosis factor receptor 1 results in activation of nuclear factor kappa B and release of interleukin-6 in bone infection. Microbiology 159, 147–154 (2013).
Yao, J., Zhong, J., Fang, Y., Geisinger, E., Novick, R.P. & Lambowitz, A.M. Use of targetrons to disrupt essential and nonessential genes in Staphylococcus aureus reveals temperature sensitivity of Ll.LtrB group II intron splicing. RNA 12, 1271–1281 (2006).
Pynnonen, M., Stephenson, R.E., Schwartz, K., Hernandez, M. & Boles, B.R. Hemoglobin promotes Staphylococcus aureus nasal colonization. PLoS Pathog. 7, e1002104 (2011).
Clarke, S.R., Andre, G., Walsh, E.J., Dufrene, Y.F., Foster, T.J. & Foster, S.J. Iron-regulated surface determinant protein A mediates adhesion of Staphylococcus aureus to human corneocyte envelope proteins. Infect. Immun. 77, 2408–2416 (2009).
Archer, N.K., Harro, J.M. & Shirtliff, M.E. Clearance of Staphylococcus aureus nasal carriage is T cell dependent and mediated through interleukin-17A expression and neutrophil influx. Infect. Immun. 81, 2070–2075 (2013).
Wertheim, H.F. et al. Key role for clumping factor B in Staphylococcus aureus nasal colonization of humans. PLoS Med. 5, e17 (2008).
Winstel, V. et al. Wall teichoic acid glycosylation governs Staphylococcus aureus nasal colonization. MBio 6, e00632 (2015).
Weidenmaier, C. et al. Role of teichoic acids in Staphylococcus aureus nasal colonization, a major risk factor in nosocomial infections. Nat. Med. 10, 243–245 (2004).
Botelho-Nevers, E. et al. Are the risk factors associated with Staphylococcus aureus nasal carriage in patients the same than in healthy volunteers? Data from a cohort of patients scheduled for orthopedic material implantation. Am. J. Infect. Control 42, 1121–1123 (2014).
Olsen, K. et al. Staphylococcus aureus nasal carriage is associated with serum 25-hydroxyvitamin D levels, gender and smoking status. The Tromso Staph and Skin Study. Eur. J. Clin. Microbiol. Infect. Dis. 31, 465–473 (2012).
van Cleef, B.A. et al. Livestock-associated MRSA in household members of pig farmers: transmission and dynamics of carriage, a prospective cohort study. PLoS One 10, e0127190 (2015).
Eko, K.E., Forshey, B.M., Carrel, M., Schweizer, M.L., Perencevich, E.N. & Smith, T.C. Molecular characterization of methicillin-resistant Staphylococcus aureus (MRSA) nasal colonization and infection isolates in a Veterans Affairs hospital. Antimicrob. Resist. Infect. Control 4, 10 (2015).
Lee, Y.J. et al. Impact of active screening for methicillin-resistant Staphylococcus aureus (MRSA) and decolonization on MRSA infections, mortality, and medical cost: a quasi-experimental study in surgical intensive care unit. Crit. Care 19, 143 (2015).
Bode, L.G. et al. Preventing surgical-site infections in nasal carriers of Staphylococcus aureus. N. Engl. J. Med. 362, 9–17 (2010).
Iwase, T. et al. Staphylococcus epidermidis Esp inhibits Staphylococcus aureus biofilm formation and nasal colonization. Nature 465, 346–349 (2010).
Lamy Filho, F. et al. Effect of maternal skin-to-skin contact on decolonization of methicillin-oxacillin-resistant Staphylococcus in neonatal intensive care units: a randomized controlled trial. BMC Pregnancy Childbirth 15, 63 (2015).
Zaidi, T., Zaidi, T., Cywes-Bentley, C., Lu, R., Priebe, G.P. & Pier, G.B. Microbiota-driven immune cellular maturation is essential for antibody-mediated adaptive immunity to Staphylococcus aureus infection in the eye. Infect. Immun. 82, 3483–3491 (2014).
Naik, S. et al. Commensal-dendritic-cell interaction specifies a unique protective skin immune signature. Nature 520, 104–108 (2015).
Pauli, N.T. et al. Staphylococcus aureus infection induces protein A-mediated immune evasion in humans. J. Exp. Med. 211, 2331–2339 (2014).
Burian, M., Wolz, C. & Goerke, C. Regulatory adaptation of Staphylococcus aureus during nasal colonization of humans. PLoS One 5, e10040 (2010).
Van Belkum, A. et al. Coagulase and protein A polymorphisms do not contribute to persistence of nasal colonisation by Staphylococcus aureus. J. Med. Microbiol. 46, 222–232 (1997).
van Belkum, A. & Schrenzel, J. Staphylococcus aureus: the innocent culprit? Infect. Genet. Evol. 21, 509 (2014).
Verkaik, N.J. et al. Anti-staphylococcal humoral immune response in persistent nasal carriers and noncarriers of Staphylococcus aureus. J. Infect. Dis. 199, 625–632 (2009).
Holtfreter, S. et al. Human immune proteome in experimental colonization with Staphylococcus aureus. Clin. Vaccine Immunol. 16, 1607–1614 (2009).
van Belkum, A. et al. Reclassification of Staphylococcus aureus nasal carriage types. J. Infect. Dis. 199, 1820–1826 (2009).
Thammavongsa, V., Rauch, S., Kim, H.K., Missiakas, D.M. & Schneewind, O. Protein A-neutralizing monoclonal antibody protects neonatal mice against Staphylococcus aureus. Vaccine 33, 523–526 (2015).
Delfani, S., Mohabati Mobarez, A., Imani Fooladi, A.A., Amani, J. & Emaneini, M. Protection of mice against Staphylococcus aureus infection by a recombinant protein ClfA-IsdB-Hlg as a vaccine candidate. Med. Microbiol. Immunol. (2015) e-pub ahead of print.
Shopsin, B. et al. Evaluation of protein A gene polymorphic region DNA sequencing for typing of Staphylococcus aureus strains. J. Clin. Microbiol. 37, 3556–3563 (1999).
Harmsen, D. et al. Typing of methicillin-resistant Staphylococcus aureus in a university hospital setting by using novel software for spa repeat determination and database management. J. Clin. Microbiol. 41, 5442–5448 (2003).
Shinji, H., Sakurada, J., Seki, K., Murai, M. & Masuda, S. Different effects of fibronectin on the phagocytosis of Staphylococcus aureus and coagulase-negative staphylococci by murine peritoneal macrophages. Microbiol. Immunol. 42, 851–861 (1998).
Shinji, H. et al. Role of fibronectin-binding proteins A and B in in vitro cellular infections and in vivo septic infections by Staphylococcus aureus. Infect. Immun. 79, 2215–2223 (2011).
Monk, I.R., Shah, I.M., Xu, M., Tan, M.W. & Foster, T.J. Transforming the untransformable: application of direct transformation to manipulate genetically Staphylococcus aureus and Staphylococcus epidermidis. MBio 3, e00277–11 (2012).
Jena, P. et al. Azurophil granule proteins constitute the major mycobactericidal proteins in human neutrophils and enhance the killing of mycobacteria in macrophages. PLoS One 7, e50345 (2012).
Boyum, A. Isolation of mononuclear cells and granulocytes from human blood. Isolation of monuclear cells by one centrifugation, and of granulocytes by combining centrifugation and sedimentation at 1 g. Scand. J. Clin. Lab. Invest. Suppl. 97, 77–89 (1968).
Acknowledgements
A.M.C. was supported by NIH grant AI 060753 as well as internal funds from UCF. We thank Ryan P. Lamers, Philip Adams, and Vanathy Paramanandam for technical assistance, and Ian R. Monk and Timothy J. Foster (Trinity College Dublin) for providing the E. coli strain DC10B. We are grateful to all volunteers who donated swabs, nasal fluid, and neutrophils and participated in the nasal inoculation studies.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declared no conflict of interest.
Additional information
SUPPLEMENTARY MATERIAL is linked to the online version of the paper
Rights and permissions
About this article
Cite this article
Cole, A., Muthukrishnan, G., Chong, C. et al. Host innate inflammatory factors and staphylococcal protein A influence the duration of human Staphylococcus aureus nasal carriage. Mucosal Immunol 9, 1537–1548 (2016). https://doi.org/10.1038/mi.2016.2
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1038/mi.2016.2
This article is cited by
-
Staphylococcus aureus Evasion of Host Immunity in the Setting of Prosthetic Joint Infection: Biofilm and Beyond
Current Reviews in Musculoskeletal Medicine (2018)
-
The commensal lifestyle of Staphylococcus aureus and its interactions with the nasal microbiota
Nature Reviews Microbiology (2017)