Abstract
Tuberculosis (TB) vaccine development has focused largely on targeting T helper type 1 (Th1) cells. However, despite inducing Th1 cells, the recombinant TB vaccine MVA85A failed to enhance protection against TB disease in humans. In recent years, Th17 cells have emerged as key players in vaccine-induced protection against TB. However, the exact cytokine and immune requirements that enable Th17-induced recall protection remain unclear. In this study, we have investigated the requirements for Th17 cell-induced recall protection against Mycobacterium tuberculosis (Mtb) challenge by utilizing a tractable adoptive transfer model in mice. We demonstrate that adoptive transfer of Mtb-specific Th17 cells into naive hosts, and upon Mtb challenge, results in Th17 recall responses that confer protection at levels similar to vaccination strategies. Importantly, although interleukin (IL)-23 is critical, IL-12 and IL-21 are dispensable for protective Th17 recall responses. Unexpectedly, we demonstrate that interferon-γ (IFN-γ) produced by adoptively transferred Th17 cells impairs long-lasting protective recall immunity against Mtb challenge. In contrast, CXCR5 expression is crucial for localization of Th17 cells near macrophages within well-formed B-cell follicles to mediate Mtb control. Thus, our data identify new immune characteristics that can be harnessed to improve Th17 recall responses for enhancing vaccine design against TB.
Similar content being viewed by others
INTRODUCTION
Mycobacterium tuberculosis (Mtb), the etiological agent of tuberculosis (TB), infects one-third of the world’s population, causing clinical pulmonary TB in ∼9 million people and resulting in ∼1.3 million deaths per year.1 The variable efficacy of the current TB vaccine Mycobacterium bovis Bacillus Calmette–Guérin (BCG) against pulmonary TB, along with the recent emergence of drug-resistant Mtb strains, has prompted the search for novel vaccines for TB.2 The paradigm for TB vaccine development in the past has focused on targeting enhancement of interferon-γ (IFN-γ) secretion in T cells to mediate early macrophage activation and bacterial killing.3 However, despite induction of high levels of IFN-γ production in adults and infants,4, 5 the recombinant TB vaccine MVA85A tested in human clinical trials failed to protect against TB disease in infants.6 These studies highlight the importance of exploring new and more effective pathways to improve vaccine-induced immunity against TB.
In recent years, T helper type 17 (Th17) cells have emerged as one of the primary effector cells that mediate inflammation in autoimmune diseases.7 On the other hand, Th17 cells are critical for mediating immunity against extracellular bacterial and fungal pathogens8 as well as in vaccine-induced protection against several mucosal pathogens,9 including Mtb.10 Indeed, our studies were among the earliest to show that parenteral vaccine-induced Th17 cells populate the lung and respond rapidly to Mtb infection, thus enabling Mtb containment.11 More recently, we have shown that mucosal vaccine-driven protection is dependent on interleukin 17 (IL-17) production by Th17 cells, subsequent production of chemokines, and localization of T cells and B cells for formation of organized ectopic B-cell follicles facilitating activation of Mtb-infected macrophages.12 In addition, several vaccination strategies have been shown to promote potent, long-lasting Th17 responses. For example, immunization with two tuberculosis fusion proteins, H1 and H28, in adjuvant CAF0113 and vaccination with the recombinant BCG strain rBCGΔureC:Hly in mice14 both induce strong Th17 responses that are associated with superior protection to Mtb challenge. However, despite the emerging consensus that Th17 cells are critical for vaccine-induced immunity against TB, the exact cytokine and immune requirements that enable Th17-induced recall protection upon Mtb challenge remain unclear. Delineating the immune characteristics of Th17 cells that mediate recall protection against TB is critical for targeting Th17 responses for development of improved vaccines against TB.
In this study, we have investigated the requirements for Th17 cell-induced recall protection against Mtb challenge by utilizing a tractable adoptive transfer model in mice infected with Mtb. Using this model, our studies demonstrate that adoptive transfer of Mtb-specific Th17 cells into naive hosts and upon Mtb challenge leads to early cytokine production and confers protection at levels similar to that seen with vaccination strategies. In addition, our new results demonstrate that protective Th17 recall responses are IL-12 and IL-21 independent, but completely IL-23 dependent. Surprisingly, we show that the ability to coproduce IFN-γ by Th17 cells is detrimental to long-lasting protective recall immunity against Mtb challenge, suggesting that efforts to limit IFN-γ production rather than enhance IFN-γ production in vaccine-induced T cells may improve efficacy of TB vaccines. Our data also demonstrate that Th17-induced protection is dependent on expression of CXCR5 for strategic localization of T cells within and around organized B-cell follicles, thus mediating efficient macrophage activation and Mtb control. Given the urgency for the development of safe and effective vaccines against TB, our data presented here identify new immune mechanisms that can be harnessed to improve recall responses by Th17 cells for vaccine design against TB.
RESULTS
Th17 recall responses mediate Mtb control in an IL-12 and IL-21-independent but IL-23-dependent manner
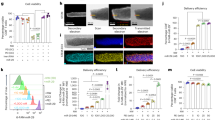
Th17 recall responses are associated with vaccine-induced protection against TB,11, 12, 15, 16 but the cytokines and factors that are required for effective Th17 recall responses in vivo upon Mtb challenge are not well described. In order to study the requirements for Th17 recall responses in vivo upon Mtb challenge, we isolated naive CD4+ T cells from ESAT-6 T cell receptor (TCR) transgenic (Tg) mice and differentiated them in vitro under Th1- or Th17-skewing conditions. As expected, Th17 cells produced high levels of IL-17A (IL-17) and IL-21, but not IFN-γ, when compared with Th1 cells that produced IFN-γ (Supplementary Figure S1A,B online). In vitro differentiated Th17 cells were then adoptively transferred into C57BL/6 (B6) hosts and following a period of rest, mice were challenged with low doses of aerosolized Mtb H37Rv. We found that adoptive transfer of Th17 cells into B6 hosts resulted in lower lung bacterial burden when compared with control B6 mice that did not receive cells (Figure 1a). Importantly, the level of protection conferred by adoptive transfer of Th17 cells was comparable to parenteral or mucosal immunization with ESAT-61–20, an immunodominant Mtb antigen, known to confer vaccine-induced protection upon Mtb challenge11, 12, 15 (Figure 1a). Importantly, upon Mtb challenge, early IL-17 antigen-specific recall responses were observed in mice that received Th17 cells and mice that were previously vaccinated when compared with unvaccinated mice that did not exhibit early IL-17 responses (Figure 1b). In addition, mucosally vaccinated mice had increased recall protection when compared with parenterally vaccinated mice (Figure 1a) and this coincided with increased accumulation of early IL-17 responses in the lung upon Mtb challenge (Figure 1b). These data suggest that the recall response mediated by adoptive transfer of in vitro generated Th17 cells was by itself sufficient to control Mtb to levels comparable to prior vaccination. Thus, our model of adoptive transfer of antigen-experienced Th17 cells is a useful tool to study the factors required for effective Th17 recall responses in vivo upon Mtb challenge.
T helper type 17 (Th17) recall responses mediate Mycobacterium tuberculosis (Mtb) control through an interleukin (IL)-12 and IL-21-independent but IL-23-dependent mechanism. (a) C57BL/6 mice were vaccinated with ESAT-61–20 peptide in monophosphoryl lipid A/trehalose dicorynomycolate/dimethyl dioctadecylammonium bromide (MPL/TDM/DDA) adjuvant as described, or mucosally vaccinated with ESAT-61–20 in LT-IIb holotoxin mucosal adjuvant rested for 30 days, and infected with ∼100 colony-forming units (CFUs) Mtb H37Rv. Naive unvaccinated mice (UnVac) were included as controls. Alternatively, mice received 2 × 106 ESAT-6 T cell receptor (TCR) transgenic (Tg) Th17 cells intravenously (i.v.), were rested, and aerosol infected with ∼100 CFUs Mtb H37Rv. Lung bacterial burden at 40 days post infection (DPI) was determined by plating on 7H11 agar plates. (b) IL-17-producing ESAT-6-specific CD4+ T cells were determined via peptide-driven enzyme-linked immunospot (ELISpot) on day 15 after Mtb challenge. C57BL/6 (B6), IL-12p35−/−, IL-21−/−, or IL-23p19−/− mice were left untreated (−) or received 2 × 106 ESAT-6 TCR Tg Th17 cells by i.v. route, and were aerosol challenged with 100 CFUs Mtb H37Rv. (c, f, i) Lung bacterial burden at 40 DPI and (d, g, j) the number of interferon-γ (IFN-γ) or (e, h, k) IL-17-producing ESAT-6-specific CD4+ T cells were determined via peptide-driven ELISpot. The data points represent the mean (±s.d.) of values from 4 to 8 mice. *P≤0.05, **P≤0.01, ***P≤0.001.
Th17 cell differentiation occurs in the presence of the polarizing cytokines IL-6 and transforming growth factor-β.17, 18 In addition, IL-23 mediates commitment to the Th17 subset,19 whereas autocrine production of IL-21 induces Th17 differentiation.20 Although much is understood about how Th17 cells differentiate in vitro and during primary immune responses, the requirements for Th17 recall responses in vivo, specifically upon Mtb recall challenge, have not been well characterized. Thus, using the Th17 cell adoptive transfer model, we then addressed the role of IL-12, IL-23, and IL-21 in mediating effective Th17 recall responses and conferring protection upon Mtb challenge. IL-12 is a polarizing cytokine required for differentiation of Th1 responses,21, 22 and is thus critical for protective immunity against TB.23 Accordingly, IL-12p35−/− control mice were more susceptible to Mtb challenge when compared with B6 control mice (Figure 1c). Similar to B6 mice that received Th17 cells (Figure 1a), adoptive transfer of Th17 cells into IL-12p35−/− mice resulted in reduction in lung Mtb burden (Figure 1c), suggesting that although IL-12 is required for protective primary immunity against TB, IL-12 is not required for protective Th17 recall responses upon Mtb challenge. Interestingly, IL-12p35−/− mice that received Th17 cells also exhibited increased ESAT-6-specific IFN-γ-producing T cells (Figure 1d) and increased ESAT-6-specific IL-17-producing cells when compared with cytokine production in naive B6 mice (Figure 1e). IL-21 is a cytokine that is involved in the induction of Th17 cells.20 As previously described,24 IL-21−/− mice challenged with Mtb did not have increased bacterial burden when compared with B6 control mice. Interestingly, Th17 adoptive transfer into IL-21−/− mice improved Mtb control when compared with IL-21−/− mice that did not receive cells (Figure 1f). These results indicate a dispensable role for IL-21 in both primary immunity24 and protective Th17 recall responses in TB. The protection afforded in the IL-21−/− mice coincided with generation of efficient Mtb-specific IFN-γ-producing (Figure 1g) and IL-17-producing (Figure 1h) T-cell responses. Interestingly, when we transferred in vitro differentiated Th17 cells into IL-23p19−/− mice and evaluated their ability to confer protection upon Mtb challenge, we found that IL-23 was required for Th17-induced protection. As shown before,25 IL-23p19−/− control mice are not more susceptible to Mtb infection when compared with B6 control mice. However, IL-23p19−/− mice that received Th17 cells had comparable lung bacterial burdens to IL-23p19−/− mice that did not receive T cells (Figure 1i), suggesting that despite a dispensable role for IL-23 in primary immunity to Mtb infection, IL-23 has a critical role in Th17 recall response-mediated protection against Mtb infection. Incidentally, IL-23p19−/− mice that received Th17 cells and did not mediate early Mtb control also exhibited defective IL-17 responses (Figure 1k), although ESAT-6-specific IFN-γ responses were robust and at levels comparable to B6 Mtb-infected mice (Figure 1j). These data together project a critical role for IL-23, but not IL-12 or IL-21, in Th17 recall responses in response to Mtb infection. In addition, these data clearly show that Th17 cells generated in vitro in the presence of IL-23 still required the presence of IL-23 for effective Th17 recall response following Mtb challenge.
Transferred Th17 recall responses occur early upon Mtb challenge and produce both IFN-γ and IL-17
To further understand the immune features of Th17 recall responses, we tracked Thy1.1+ Th1 or Th17 cells upon adoptive transfer in B6 Thy1.2+ congenic hosts. We found that adoptively transferred Th17 cells preferentially migrated to, and were retained in the lung compartment, when compared with similarly transferred Th1 cells (Figure 2a,b). To further delineate the early kinetics of Th17 recall responses upon Mtb challenge, we isolated naive undifferentiated Thy1.1+ Th0 cells or in vitro generated Thy1.1+ Th17 cells or Th1 cells from ESAT6 TCR Tg mice and adoptively transferred cells into B6 Thy1.2+ congenic mice, rested them, and then challenged them with Mtb, and early Thy1.1+ Mtb-specific recall responses were assessed on days 10, 12, and 14 after Mtb infection (gating strategy shown in Figure 2c). Consistent with our observation that Th17 cells accumulate and are retained in the lung upon adoptive transfer and before Mtb challenge (Figure 2a,b), we observed that the number of Th17 cells increased in the lungs between days 12 and 14 after Mtb challenge (Figure 2c,d). This was in contrast to B6 mice that received Th0 cells or Th1 cells, where significantly fewer ESAT-6-specific Th cells were detected in the lungs at these early time points (Figure 2d). This is consistent with previous studies that have shown that Mtb-specific Th0 cells take 13–18 days to accumulate in the Mtb-infected lungs.26, 27, 28 Interestingly, in vitro differentiated Th17 cells that were primarily IL-17 producers at the time of transfer (Supplementary Figure S1A) produced IFN-γ following Mtb challenge, with or without IL-17 expression (Figure 2e). The majority of the adoptively transferred Mtb-specific Th17 cells were activated as detected by expression of CD44 (Figure 2c), suggesting that the ability to coproduce IFN-γ is not because of in vivo priming of undifferentiated adoptively transferred T cells. These data together show that antigen-experienced Mtb-specific Th17 cells accumulate early in the lungs following Mtb challenge when compared with naive Th0 cells or antigen-experienced Th1 cells. In addition, our new data demonstrate that in vitro differentiated Th17 recall responses that did not produce much IFN-γ at the time of adoptive transfer (Supplementary Figure S1), upon transfer can confer protection to Mtb challenge (Figure 1a), and can acquire the ability to coproduce IFN-γ during early recall responses. Furthermore, we adoptively transferred ESAT6 TCR Tg Th0 cells into B6 Thy1.2 mice that were then parenterally vaccinated, rested, and then challenged with Mtb. Similar to the data with the adoptive transfer of Th17 cells and Mtb challenge, we found that vaccine-induced cells also accumulated between days 10 and 13 in the lung and coproduced IFN-γ and IL-17 (Figure 2f). Thus, our data show that Th17 recall responses in the adoptive transfer model occur early upon Mtb challenge, produce both IFN-γ and IL-17, and compare well with natural models of vaccine-induced recall responses.
T helper type 17 (Th17) recall responses occur early and can produce both interferon-γ (IFN-γ) and interleukin-17 (IL-17). 2 × 106 ESAT-6 T cell receptor (TCR) transgenic (Tg) Thy1.1+ Th0, Th1, or Th17 cells were transferred intravenously (i.v.) into C57BL/6 (Thy1.2+ congenic background) and lung cell suspensions were prepared on day 7 after transfer. (a) Gating showing accumulation of adoptively transferred cells in lungs day 7 and (b) total numbers of lung-resident Th1 or Th17 adoptively transferred cells calculated. In a separate experiment, mice receiving adoptively transferred cells were rested and then infected with ∼100 colony-forming units (CFUs) Mycobacterium tuberculosis (Mtb) H37Rv via aerosol and lung cell suspensions analyzed at 10, 12, and 14 days post infection (DPI) by flow cytometry for cytokine production. (c) Gating strategy for flow cytometric analysis. Numbers of (d) CD3+CD4+CD44+Thy1.1+ and (e) cytokine-producing cells were determined. (f) In a separate experiment, B6 Thy1.2+ mice received 2 × 106 ESAT-6 TCR Tg Th0 cells, were vaccinated with ESAT-61–20 peptide in monophosphoryl lipid A/trehalose dicorynomycolate/dimethyl dioctadecylammonium bromide (MPL/TDM/DDA) adjuvant, rested, and then infected with ∼100 CFUs Mtb via aerosol and cytokine-producing cells were determined in the lung at early time points. The data points represent the mean (±s.d.) of values from five mice. *P≤0.05.
IFN-γ production by Th17 cells limits recall protection against Mtb challenge
Given the ability of adoptively transferred Th17 antigen-experienced cells to coproduce IFN-γ (Figure 2e), we next sought to determine whether IFN-γ or IL-17 production by Th17 cells was mediating the control upon Mtb challenge. Thus, we generated in vitro differentiated Th17 cells from ESAT-6 TCR Tg IFN-γ-deficient and IFN-γ-sufficient ESAT6 TCR Tg mice and adoptively transferred similar numbers of ESAT-6 TCR Tg IFN-γ-deficient or IFN-γ-sufficient Th17 cells into IL-12p35−/− mice that lack IFN-γ responses (Figure 1d) and determined lung bacterial burden following Mtb challenge. Surprisingly, not only was IFN-γ dispensable for Th17-induced protection, but IFN-γ deficiency in Th17 cells further improved protection in IL-12p35−/− mice when compared with adoptive transfer of IFN-γ-sufficient Th17 cells (Figure 3a). Our recent work has demonstrated a critical role for IL-17 in induction of CXCL-13 and formation of ectopic lymphoid structures in the lung for early Mtb control.24, 29 Consistent with these findings, we found that adoptive transfer of IFN-γ-deficient Th17 cells more efficiently induced localized CXCL-13 protein expression within lymphoid follicles (Figure 3b) and triggered the formation of well-organized B cell follicles in the lungs (Figure 3b,c) when compared with lungs of IL-12p35−/− mice that received IFN-γ-sufficient Th17 cells. Increased ectopic B-cell follicle formation also coincided with a reduction in T-cell perivascular cuffing (Figure 3d), indicative of migration of T cells to areas containing Mtb-infected macrophages.24 Importantly, upon Mtb challenge, we found that the IL-17 antigen-specific responses detected in IL-12p35−/− mice that received either ESAT-6 TCR Tg IFN-γ-deficient and IFN-γ-sufficient Th17 cells were comparable (Figure 3e). These findings together project that not only is IFN-γ production by Th17 cells dispensable for T-cell recall immunity against TB, but also in fact the presence of early IFN-γ is detrimental to recall immunity against Mtb challenge.
Interferon-γ (IFN-γ) production in T helper type 17 (Th17) cells decreases the potency of recall protection against Mycobacterium tuberculosis (Mtb) challenge. Th17 cells were differentiated in vitro from IFN-γ-sufficient and IFN-γ-deficient ESAT-6 T cell receptor (TCR) transgenic (Tg) mice. IL-12p35−/− mice received 2 × 106 ESAT-6 TCR Tg Th17- or IFN-γ-deficient Th17 cells intravenously (i.v.) or were left untreated (−), rested, and aerosol infected with Mtb. (a) Lung bacterial burden at 40 days post infection (DPI) was determined by plating on 7H11 agar plates. (b) Formalin-fixed, paraffin-embedded serial lung sections from the above groups were processed for immunofluorescence using antibodies specific for CXCL-13 or B220 and CD3. (c) The average (Avg.) size of B-cell follicles and (d) the average (Avg) area occupied by perivascular T-cell cuffing were calculated using the morphometric tool of the Zeiss Axioplan microscope. (e) Interleukin (IL)-17-producing ESAT-6-specific CD4+ T cells were determined via peptide-driven enzyme-linked immunospot (ELISpot). The data points represent the mean (±s.d.) of values from 5 to 8 mice. *P≤0.05, **P≤0.01, ***P≤0.001, NS, not significant.
M. bovis BCG, the current vaccine against TB, confers protection against pediatric cases of disseminated TB, but its efficacy against adult pulmonary TB ranges from 0 to 80%. Defining the requirements for induction of long-lasting protective immunity is therefore a key parameter in the development of more efficacious vaccines against TB. Thus, we next determined whether Th17-induced protection observed in the IL-12p35−/− mice (Figure 3) was long lasting and whether the absence of IFN-γ in adoptively transferred cells conferred any advantage to Mtb control in hosts. Accordingly, we found that although IL-12p35−/− mice that received Th17 cells had short-term protection (Figure 3a), the IL-12p35−/− hosts that received Th17 cells lost the protective effects at ∼100 days after challenge (Figure 4a). This was despite the fact that IL-12p35−/− hosts that received Th17 cells had significantly larger B-cell follicles (Figure 4b,c), increased localization of CXCL13 protein within follicles (Figure 4b), and decreased T-cell perivascular cuffing (Figure 4b,d) when compared with IL-12p35−/− mice that did not receive Th17 cells. Importantly, transfer of IFN-γ-deficient Th17 cells conferred sustained long-term protection in IL-12p35−/− mice (Figure 4a), and this coincided with significantly larger ectopic lymphoid follicles (Figure 4b,c) and decreased T-cell perivascular cuffing (Figure 4d), indicating that the establishment of a certain threshold B-cell follicle size may be required for long-lasting Mtb containment. These data together for the first time suggest that the ability to produce IFN-γ by Th17 cells may compromise long-lasting protective ability of T-cell recall responses following Mtb challenge.
Interferon-γ (IFN-γ)-deficient adoptively transferred T helper type 17 (Th17) cells induce long-lasting protection against Mycobacterium tuberculosis (Mtb) challenge. IL-12p35−/− mice received 2 × 106 ESAT-6 T cell receptor (TCR) transgenic (Tg) Th17 or IFN-γ-deficient Th17 cells intravenously (i.v.) or were left untreated, rested, and aerosol infected with Mtb. (a) Lung bacterial burden at 100 days post infection (DPI) was determined by plating on 7H11 agar plates. (b) Formalin-fixed, paraffin-embedded serial lung sections from the above groups were processed for immunofluorescence using antibodies specific for CXCL-13, and B220, and CD3. (c) The average (Avg.) size of B-cell follicles and (d) the average area occupied by perivascular T-cell cuffing were calculated using the morphometric tool of the Zeiss Axioplan microscope. The data points represent the mean (±s.d.) of values from 5 to 8 mice. **P≤0.01, ***P≤0.001.
CXCR5 expression on adoptively transferred Th17 cells is critical for the protective recall responses against TB
We have recently described a role for early vaccine-induced IL-17 in mediating CXCL-13 expression that resulted in localization of CXCR5+ cytokine-producing T cells near Mtb-infected macrophages, an event crucial for optimal Mtb control.12 The correct localization of T cells expressing CXCR5 within the lung parenchyma results in formation of lymphoid structures within TB granulomas that is required for activation of infected macrophages for control of Mtb.24 We have found that measurement of B-cell follicle formation can be used as an effective readout of T-cell localization within TB granulomas and Mtb control.12, 24, 30, 31 Using Cxcr5−/− ESAT-6 TCR Tg Th17 cells, we next determined whether expression of this receptor was required for Th17-driven protection in recall responses to Mtb challenge. Thus, we adoptively transferred either no cells, Th17 cells, IFN-γ-deficient Th17 cells, or CXCR5-deficient Th17 cells into B6 mice and following a period of rest, challenged mice with low doses of Mtb. We found that similar to our data with IL-12p35−/− mice that received IFN-γ-deficient Th17 cells (Figures 3 and 4), absence of IFN-γ in Th17 cells conferred better recall protection even in B6 mice when compared with B6 mice that received IFN-γ-sufficient Th17 cells (Figure 5a). Most importantly, we found that B6 mice that received CXCR5-deficient Th17 cells exhibited impaired Mtb control, whereas B6 mice that received CXCR5-sufficient Th17 cells conferred protection (Figure 5a), suggesting that expression of CXCR5 on Th17 cells is critical for the protective recall responses mediated by Th17 cells. Cxcr5−/− mice do not form ectopic lymphoid structures and are more susceptible to Mtb challenge.24 Thus, we next addressed whether adoptive transfer of CXCR5-sufficient Th17 cells is sufficient to rescue ectopic structure formation and confer protective recall responses in Cxcr5−/− mice. We found that adoptive transfer of ESAT-6 TCR Tg Th17 cells into Cxcr5−/− mice resulted in improved Mtb control (Figure 5b), suggesting that presence of CXCR5 on adoptively transferred Th17 cells was necessary to mediate recall responses in CXCR5-deficient hosts. This was associated with improved B-cell follicle formation (Figure 5c and e), and diminished T-cell perivascular cuffing (Figure 5d,e). Taken together, these results indicate that CXCR5 expression on Th17 cells during recall responses is necessary for correct localization of T cells within the lung parenchyma near infected macrophages to mediate Mtb control.
CXCR5 expression on T helper type 17 (Th17) cells is critical to mediate protective recall response following Mycobacterium tuberculosis (Mtb) challenge. B6 mice received 2 × 106 ESAT-6 T cell receptor (TCR) transgenic (Tg) wild-type (WT), interferon-γ (IFN-γ)-deficient or CXCR5-deficient Th17 cells intravenously (i.v.) or were left untreated, rested, and aerosol infected with Mtb. (a) Lung bacterial burden at 40 days post infection (DPI) was determined by plating on 7H11 agar plates. B6 or Cxcr5−/− mice received 2 × 106 ESAT-6 TCR Tg Th17 cells i.v. or were left untreated, rested, and aerosol infected with Mtb. (b) Lung bacterial burden at 40 DPI was determined by plating on 7H11 agar plates. Formalin-fixed, paraffin-embedded serial lung sections from the above groups were processed for immunofluorescence using antibodies specific for B220, CD3, and IgG. (c) The average (Avg.) size of B-cell follicles and (d) the average area occupied by perivascular T-cell cuffing were calculated from (e) B220-, CD3-, and IgG-stained lung sections. The data points represent the mean (±s.d.) of values from five mice. *P≤0.05, **P≤0.01, ***P≤0.001. NS, not significant.
DISCUSSION
The recent emergence of drug-resistant Mtb strains,2 the spread of the HIV-TB coepidemic,2 and the disappointing efficacy data from the recent human clinical trials with MVA85A6 together highlight the big gaps in our understanding of how protective recall responses function during Mtb challenge. If we want to reach the deadline of elimination of TB as a public health problem by 2050, the development of safe and more effective vaccines against TB is urgent and an area of top priority. Early studies projected Th1 responses and the cytokine IFN-γ in primary protection against TB,32 where it plays a role in the potentiation of macrophage-killing mechanisms. Thus, most vaccines in the past have used induction of IFN-γ responses as a correlate for vaccine efficacy.10 However, recent studies have demonstrated that although vaccine-induced protection occurs in the absence of IFN-γ, vaccine-induced recall protection is lost in the absence of IL-17.11, 12, 15, 16 Thus, it is now becoming clear that targeting Th17 cells rather than Th1 cells to improve vaccine-induced immunity may enhance the efficacy of future vaccine strategies against TB.10, 33 However, in order to improve Th17 recall responses, we need to first understand the immune characteristics of Th17 recall responses that confer protection against Mtb challenge. Thus, using a Th17 cell transfer model, we have identified that Th17 recall responses are dependent on IL-23, whereas endogenous IL-12 and IL-21 are not required for effective Th17 recall responses. In addition, we demonstrate that upon Mtb challenge, Th17 recall responses appear earlier than naive or Th1 cells and acquire the ability to produce IFN-γ. Importantly, the ability to produce IFN-γ is in fact detrimental to long-lasting protective recall responses against TB, whereas expression of CXCR5 by Th17 cells is necessary to mediate strategic positioning of Th17 recall responses within the lung to promote early Mtb control. Thus, identification of new immune characteristics of Th17 cells, as described in this paper, are critical to harness novel pathways to enhance Th17 recall responses for design of more effective vaccines against TB.
Our new data show that adoptive transfer of ESAT-6-specific Th17 cells into naive hosts followed by a period of rest and Mtb challenge results in similar levels of protection as prior vaccination with ESAT-61–20 antigen in adjuvant. IL-23 is an important factor in differentiation of effector Th17 cells.19 Accordingly, our early work showed that IL-23p19−/− mice vaccinated with parenteral Mtb antigen vaccine had reduced priming of Th17 responses and demonstrated loss of vaccine-induced protection upon Mtb challenge, suggesting that IL-23 was critical for initiation of vaccine-induced Th17 responses.11 However, because IL-23p19−/−-vaccinated hosts have defects in initiation of Th17 responses, we cannot use IL-23-deficient hosts to specifically address whether IL-23 has a role to play in Th17 recall responses upon Mtb challenge. Thus, our studies in this paper demonstrate for the first time that Th17 cells primed in vitro in the presence of IL-23 also require the presence of IL-23 for effective Th17 recall responses that contribute to protection upon Mtb challenge. This is consistent with a recent study that showed an important role for IL-23 in activation of memory Th17 cells in a model of experimental autoimmune encephalomyelitis.34 Interestingly, immune pathways that are important for primary immunity against TB such as IL-12 and IFN-γ are dispensable for Th17 recall protective responses. Thus, our data show a critical role for IL-23, but not IL-12 and IL-21, in activation of Th17 recall responses for protection against Mtb challenge, projecting IL-23 as a key target pathway in both generation of Th17 responses11 and activation of Th17 recall responses upon Mtb challenge.
Our previous data showed that parenteral vaccination induces a population of antigen-specific Th17 cells in the lungs that is required for vaccine-induced protection against Mtb challenge.11 Our current data support these findings as they show that adoptive transfer of Th17 cells also results in accumulation and persistence of Th17 cells in the lung compartment, suggesting that specific chemokine receptor expression such as CCR4 on Th17 cells11 may regulate the preferential accumulation in mucosal compartments. Consistent with these findings, improving lung Th17 populations by mucosal immunization strategies enhances protection against Mtb challenge.12, 16 Our data show that upon Mtb challenge, accumulation of Th17 recall responses occurs before accumulation of either Th1 recall responses or Th0 cells in the lungs of Mtb-infected mice, suggesting that the preferential location of Th17 cells in the mucosal compartment may serve as an advantage and improve protection upon pulmonary Mtb challenge. Interestingly, even in a mouse model of Mtb infection, drug therapy, and reinfection with Mtb, a steady increase in “memory” Th17 cells was observed early in the lungs of reinfected mice.35 In addition, although it is interesting that the early accumulation of Th17 recall responses upon Mtb challenge coincides with production of IFN-γ, this is not a completely unexpected finding. Several recent studies have projected Th17 cells as inherently plastic and have shown that they can readily acquire the ability to coproduce IFN-γ, especially in inflammatory models.34, 36, 37 In agreement with our findings, in observing long-term vaccine-induced Th17 recall responses to Mtb challenge, it was found that not only did IL-17-producing CD4+ T cells accumulate in the lung earlier than IFN-γ-producing T cells, but also IL-17-producing CD4+ T cells coproduced IFN-γ.13 However, it is surprising that adoptive transfer of IFN-γ-deficient Th17 cells into host mice results in more protective and long-lasting control of Mtb, suggesting a detrimental role for IFN-γ in recall responses against Mtb challenge. IFN-γ production has been shown to limit IL-17 production in T cells,38 and thus it is possible that IFN-γ deficiency in Th17 recall responses improves IL-17 production and downstream induction of chemokines and enhancement of Mtb control. However, our data show that IL-17 antigen-specific responses in IL-12p35−/− mice that receive IFN-γ-sufficient or IFN-γ-deficient Th17 cells are comparable following Mtb challenge, suggesting that this is likely not the mechanism by which IFN-γ-deficient Th17 cells confer improved protection. In addition, the increased protection afforded by adoptive transfer of IFN-γ-deficient Th17 cells holds true irrespective of whether IFN-γ-deficient Th17 cells are transferred into B6 mice or IL-12-deficient mice, suggesting that early IFN-γ production by Th17 recall responses is detrimental to Mtb control. This is in contrast to a study that adoptively transferred IFN-γ-deficient BCG-specific Th17 cells into immune-deficient mice where absence of IFN-γ did not improve protective outcomes in a systemic intravenous model of Mtb infection in mice.39 These results suggest that the mechanisms by which IFN-γ may limit protective recall immunity in a pulmonary model as shown in this study may be different from recall immunity in systemic infection models. Our findings are further supported by a recent study that used both experimental and mathematical approaches to demonstrate that the control of Mtb burden in the lung was not immediate after onset of IFNγ responses in the lung.40 As IFN-γ production has been conventionally used as a correlate for vaccine efficacy against TB, our findings instead project an important role for targeting IL-17 while limiting IFN-γ in vaccine design for TB, and as such needs to be explored in different preclinical vaccine models.
IL-17 induces expression of the homeostatic chemokines CCL-19 and CXCL-1341 that orchestrate the formation of ectopic B-cell follicles within inducible bronchus-associated lymphoid tissue. Similarly, our recent findings in vaccine models of TB demonstrate that early vaccine-induced Th17 cells produce IL-17 to mediate expression of CXCL-13 in lung stromal cells, enabling CXCR5-expressing T cells to localize near Mtb-infected macrophages.12 Thus, although B cells do not appear to play a role in low-dose Mtb infection,24, 42 the formation of B-cell follicles within TB granulomas is a good readout of T-cell localization, macrophage activation, and Mtb control.12, 24, 30, 31 Accordingly, our data show that effective Th17 recall responses coincide with localized CXCL-13 expression within lymphoid follicles, improved T-cell localization, and B-cell follicle formation for enhanced Mtb control, whereas CXCR5 deficiency on Th17 cells abrogates the protective effects of Th17 recall immune responses. Similarly, although IL-17 and IL-23 are dispensable for primary immunity against lab-adapted strains such as Mtb H37Rv, long-term control of Mtb is dependent on IL-23, and failure to contain Mtb infection in IL-23-deficient mice is associated with reduced B-cell follicle formation.43 More recently, we have shown that IL-17 is required for protective primary immunity and formation of B-cell follicles upon challenge with emerging clinical strains of Mtb, i.e., hypervirulent W-Beijing strains,30 suggesting that design of vaccines for emerging Mtb strains must target the generation of Th17 cells for effective long-term protection against TB. However, excess IL-17 production has been associated with severe lung pathology during TB.31, 44 Thus, optimization of antigen delivery strategies that promote the generation and maintenance of lung-resident Th17 cells, while minimizing potentially pathological effects of IL-17, should be carefully evaluated in preclinical models.
In conclusion, given the urgency for the development of safe and effective vaccines against TB, identification of immune characteristics of Th17 recall responses as described here can provide novel insights into pathways that can be targeted to improve vaccine design against TB. Our studies suggest that targeting Th17 cells, enhancing expression of CXCR5, or suppressing IFN-γ production in Th17 recall responses may all provide novel avenues and pathways to improve Th17 cell function and vaccine efficacy against TB.
METHODS
Animals. C57BL/6 (B6) animals were purchased from Taconic (Hudson, NY). IFNγ−/− mice on the B6 background were purchased from The Jackson Laboratory (Bar Harbor, ME). Early Secretory Antigenic Target-6 (ESAT-6) αβ TCR Tg mice recognize IAb/ESAT-61–20 and were provided by G. Winslow (Wadsworth Center, Albany, NY) and D. Woodland (Trudeau Institute, Saranac Lake, NY).26 The ESAT-6 TCR Tg mice were crossed and maintained on the Rag1–/– background or crossed to Thy1.1 mice for in vivo tracking experiments. ESAT-6.Rag−/− mice were further crossed to IFNγ−/− and CXCR5−/− mice to generate ESAT-6 TCR Tg mice deficient in these specific genes. IL-12p35−/−, IL-21−/−,24 and IL-23p19−/−45 mice were maintained in the animal facility either at the University of Pittsburgh or at Washington University in St Louis. Experimental mice were age and sex matched and used between the ages of 6 and 8 weeks. All mice were maintained and used in accordance with the approved University of Pittsburgh and Washington University in St Louis Institutional Animal Care and Use Committe guidelines.
Adoptive T-cell transfer and experimental infections. Naive T cells were isolated from ESAT-6 Tg mice using CD4+ (L3T4) magnetic bead sorting (Miltenyi Biotec, San Diego, CA). To generate Th17 cells, CD4+ T cells were cultured in a 1:1 ratio with bone marrow-derived dendritic cells in the presence of ESAT-61–20 peptide (10 μg ml−1), recombinant (r)-mouse IL-2 (10 U ml−1), r-mouse IL-6 (30 ng ml−1 R&D Systems, Minneapolis, MN), r-mouse IL-23 (50 ng ml−1 R&D Systems), r-human transforming growth factor-β (5 ng ml−1 R&D Systems), anti-IL-4 antibody (10 μg ml−1), and anti-IFN-γ antibody (10 μg ml−1) in Iscove’s modified Dulbecco’s medium (Life Technologies, Grand Island, NY). For Th1 cells, CD4+ T cells were cultured in a 1:1 ratio with bone marrow-derived dendritic cells in the presence of ESAT-61–20 peptide (10 μg ml−1), r-mouse IL-2 (10 U ml−1), r-mouse IL-12 (10 ng ml−1 R&D Systems), and anti-IL-4 antibody (10 μg ml−1) in Iscove’s modified Dulbecco’s medium (Life Technologies). For adoptive transfer, mice received 2 × 106 T cells by intravenous transfer, following which mice were rested for an average of 7 days before exposure to Mtb. In some experiments, cultured T cells were restimulated for 48 h in the presence of a 1:1 ratio of irradiated splenocytes and ESAT-61–20 peptide (10 μg ml−1) for analysis of cytokine production in the supernatant or by intracellular staining and flow cytometry.
The H37Rv strain of Mtb was grown in Proskauer Beck medium containing 0.05% Tween-80 to mid-log phase and frozen in 1 ml aliquots at −80 °C. For Mtb aerosol infections, animals were infected with 100 colony-forming units of bacteria using a Glas-Col airborne infection system as described previously.25 Lung bacterial burden was established by plating out organ homogenates on 7H11 agar plates.25 For vaccination, 400 μg of the immunodominant I-Ab-restricted ESAT-61–20 peptide (New England Peptide, Gardner, MA) was administered subcutaneously in the adjuvant mixture of monophosphoryl lipid A (Sigma-Aldrich, St Louis, MO), trehalose dicorynomycolate (Sigma-Aldrich), and dimethyl dioctadecylammonium bromide (Eastman Kodak, Rochester, NY) in a final volume of 200 μl and mice were challenged after a 30-day period of rest.11 ESAT-61–20 peptide (133.3 μg) was mixed with LT-IIb holotoxin (1 μg) and unanesthetized mice were mucosally vaccinated intranasally 3 times at 2-week intervals, such that the total amount of ESAT-61–20 used was 400 μg.
Lung single-cell preparation and detection of cytokine-producing cells by ELISpot assay. Lung suspensions from Mtb-infected mice were prepared as described previously11 and were used in enzyme-linked immunospot (ELISpot) assays as described below. Antigen-specific IFN-γ-producing and IL-17-producing cells were analyzed by ELISpot assay. Multi-screen HA filter plates (Millipore, Billerica, MA) were coated with anti-IFN-γ (BD Biosciences, San Jose, CA) or anti-IL-17 (R&D Systems) antibodies. Single-cell suspensions were added to the plate at a starting concentration of 1 × 105 cells per well and doubling dilutions made. Cells were cultured overnight in the presence of 1 × 106 irradiated splenocytes and 10 μg ml−1 ESAT-61–20 peptide and 10 U ml−1 recombinant mouse IL-2. The following day, biotinylated anti-IFN-γ or anti-IL-17 antibody (both from eBioscience, San Diego, CA) was added and incubated overnight. Plates were developed by incubation with streptavidin-alkaline phosphatase (Vector Labs, Burlingame, CA) for 2h, followed by incubation with NBT/BCIP (Sigma-Aldrich). Spots were enumerated using a CTL-ImmunoSpot analyzer (CTL, Shaker Heights, OH) and the frequency and total number of responding cells calculated as described before.11
Detection of cytokine-producing cells by flow cytometry. The presence of Thy1.1+ congenically labeled ESAT-6TCR Tg T cells, as well as the presence of cells producing IFN-γ and IL-17, was determined by flow cytometry. Single-cell suspensions were stimulated for 6 h in the presence of 50 ng ml−1 phorbol myristate acetate and 750 ng ml−1 ionomycin, as well as 5 μl ml−1 GolgiStop (BD Biosciences). Cells were treated with Fc Block (anti-CD16/CD32, BD Biosciences) before surface staining for CD3 (clone 500A2, AlexaFluor 700-conjugated, BD Biosciences), CD4 (clone RM4-5, Pacific Blue-conjugated, BD Biosciences), and CD44 (clone IM7, PE-Cy7-conjugated, eBiosciene). Cells were then fixed and permeabilized using the Cytofix/Cytoperm fixation permeabilization kit (BD Biosciences) before staining for IL-17 (clone TC11-18H10, PE-conjugated, BD Biosciences) and IFN-γ (clone XMG1.2, APC-conjugated, BD Biosciences). Cell staining was analyzed on an LSR Fortessa (BD Biosciences), and results were processed using FlowJo (Treestar, Ashland, OR).
Determination of protein concentration. Enzyme-linked immunosorbant assay antibody pairs were used to detect cytokine levels (DuoSet; R&D Biosystems) in cell culture supernatants.
Immunohistochemistry. Lung lobes were perfused with 10% neutral-buffered formalin and embedded in paraffin. For immunofluorescent staining, formalin-fixed lung sections were cut, immersed in xylene to remove paraffin, and then sequentially hydrated in absolute ethanol, 95% ethanol, 70% ethanol, and water. Antigens were unmasked with a DakoCytomation Target Retrieval Solution (Dako, Carpinteria, CA) and nonspecific binding was blocked with 5% (v/v) normal donkey serum and Fc block (BD Pharmingen, San Jose, CA). Endogenous biotin (Sigma-Aldrich) was neutralized by adding avidin followed by incubation with biotin. Sections were probed with anti-CD45R/B220 to detect B cells (Clone RA3-6B2, BD Pharmingen), anti-CXCL13 (AF470, R&D Systems), and anti-CD3 to detect T cells (Clone M-20, Santa Cruz Biotechnology, Santa Cruz, CA). B-cell follicles were outlined with the automated tool of the Zeiss Axioplan 2 microscope (Carl Zeiss, Thornwood, NY) and average size in μm2 was calculated as described before.12
Statistical analysis. Statistical analysis to determine differences between experimental groups was performed in GraphPad Prism 5 (Graph Pad, La Jolla, CA) using two-tailed Student’s t-test. Differences were considered significant when P≤0.05. All analyses were performed using GraphPad Prism Software.
References
Dye, C ., Glaziou, P ., Floyd, K . & Raviglione, M. Prospects for tuberculosis elimination. Annu. Rev. Public Health 34, 271–286 (2013).
Lienhardt, C ., Fruth, U . & Greco, M. The blueprint for vaccine research & development: walking the path for better TB vaccines. Tuberculosis (Edinb) 92 (Suppl 1), S33–S35 (2012).
Rook, G.A ., Dheda, K . & Zumla, A. Immune responses to tuberculosis in developing countries: implications for new vaccines. Nat. Rev. Immunol. 5, 661–667 (2005).
Hawkridge, T. et al. Safety and immunogenicity of a new tuberculosis vaccine, MVA85A, in healthy adults in South Africa. J. Infect. Dis. 198, 544–552 (2008).
Tameris, M. et al. The candidate TB vaccine, MVA85A, induces highly durable Th1 responses. PLoS One 9, e87340 (2014).
Tameris, M.D. et al. Safety and efficacy of MVA85A, a new tuberculosis vaccine, in infants previously vaccinated with BCG: a randomised, placebo-controlled phase 2b trial. Lancet 381, 1021–1028 (2013).
Zepp, J ., Wu, L . & Li, X. IL-17 receptor signaling and T helper 17-mediated autoimmune demyelinating disease. Trends Immunol. 32, 232–239 (2011).
Kolls, J.K . & Khader, S.A. The role of Th17 cytokines in primary mucosal immunity. Cytokine Growth Factor Rev. 21, 443–448 (2010).
Lin, Y ., Slight, S.R . & Khader, S.A. Th17 cytokines and vaccine-induced immunity. Semin. Immunopathol. 32, 79–90 (2010).
Griffiths, K.L . & Khader, S.A. Novel vaccine approaches for protection against intracellular pathogens. Curr. Opin. Immunol. 28, 58–63 (2014).
Khader, S.A. et al. IL-23 and IL-17 in the establishment of protective pulmonary CD4+ T cell responses after vaccination and during Mycobacterium tuberculosis challenge. Nat. Immunol. 8, 369–377 (2007).
Gopal, R. et al. Interleukin-17-dependent CXCL13 mediates mucosal vaccine-induced immunity against tuberculosis. Mucosal Immunol. 6, 972–984 (2013).
Lindenstrom, T. et al. Vaccine-induced th17 cells are maintained long-term postvaccination as a distinct and phenotypically stable memory subset. Infect. Immun. 80, 3533–3544 (2012).
Desel, C. et al. Recombinant BCG DeltaureC hly+ induces superior protection over parental BCG by stimulating a balanced combination of type 1 and type 17 cytokine responses. J. Infect Di.s 204, 1573–1584 (2011).
Gopal, R. et al. IL-23-dependent IL-17 drives Th1-cell responses following Mycobacterium bovis BCG vaccination. Eur. J. Immunol. 42, 364–373 (2012).
Griffiths, K.L. et al. Cholera toxin enhances vaccine-induced protection against Mycobacterium tuberculosis challenge in mice. PLoS One 8, e78312 (2013).
Harrington, L.E. et al. Interleukin 17-producing CD4+ effector T cells develop via a lineage distinct from the T helper type 1 and 2 lineages. Nat. Immunol. 6, 1123–1132 (2005).
Park, H. et al. A distinct lineage of CD4 T cells regulates tissue inflammation by producing interleukin 17. Nat. Immunol. 6, 1133–1141 (2005).
McGeachy, M.J. et al. The interleukin 23 receptor is essential for the terminal differentiation of interleukin 17-producing effector T helper cells in vivo. Nat. Immunol. 10, 314–324 (2009).
Zhou, L. et al. IL-6 programs T(H)-17 cell differentiation by promoting sequential engagement of the IL-21 and IL-23 pathways. Nat. Immunol. 8, 967–974 (2007).
Macatonia, S ., Doherty, T ., Knight, S . & O'Garra, A. Differential effect of IL-10 on dendritic cell-induced T cell proliferation and IFN-g production. J. Immunol. 150, 3755–3765 (1993).
Macatonia, S.E. et al. Dendritic cells produce IL-12 and direct the development of Th1 cells from naive CD4+ T cells. J. Immunol. 154, 5071–5079 (1995).
Cooper, A.M ., Magram, J ., Ferrante, J . & Orme, I.M. Interleukin 12 (IL-12) is crucial to the development of protective immunity in mice intravenously infected with mycobacterium tuberculosis. J. Exp. Med. 186, 39–45 (1997).
Slight, S.R. et al. CXCR5+ T helper cells mediate protective immunity against tuberculosis. J. Clin. Invest. 123, 712–726 (2013).
Khader, S.A. et al. IL-23 compensates for the absence of IL-12p70 and is essential for the IL-17 response during tuberculosis but is dispensable for protection and antigen-specific IFN-gamma responses if IL-12p70 is available. J. Immunol. 175, 788–795 (2005).
Reiley, W.W. et al. ESAT-6-specific CD4 T cell responses to aerosol Mycobacterium tuberculosis infection are initiated in the mediastinal lymph nodes. Proc. Natl. Acad. Sci. USA 105, 10961–10966 (2008).
Wolf, A.J. et al. Initiation of the adaptive immune response to Mycobacterium tuberculosis depends on antigen production in the local lymph node, not the lungs. J. Exp. Med. 205, 105–115 (2008).
Kang, D.D ., Lin, Y ., Moreno, J.R ., Randall, T.D . & Khader, S.A. Profiling early lung immune responses in the mouse model of tuberculosis. PLoS One 6, e16161 (2011).
Khader, S.A. et al. In a murine tuberculosis model, the absence of homeostatic chemokines delays granuloma formation and protective immunity. J. Immunol. 183, 8004–8014 (2009).
Gopal, R. et al. Unexpected role for IL-17 in protective immunity against hypervirulent Mycobacterium tuberculosis HN878 infection. PLoS Pathog. 10, e1004099 (2014).
Gopal, R. et al. S100A8/A9 proteins mediate neutrophilic inflammation and lung pathology during tuberculosis. Am. J. Respir. Crit. Care Med. 188, 1137–1146 (2013).
Cooper, A.M. et al. Disseminated tuberculosis in interferon gamma gene-disrupted mice. J. Exp. Med. 178, 2243–2247 (1993).
Henao-Tamayo, M ., Ordway, D.J . & Orme, I.M. Memory T cell subsets in tuberculosis: what should we be targeting? Tuberculosis (Edinb) 94, 455–461 (2014).
Haines, C.J. et al. Autoimmune memory T helper 17 cell function and expansion are dependent on interleukin-23. Cell Rep. 3, 1378–1388 (2013).
Henao-Tamayo, M. et al. A mouse model of tuberculosis reinfection. Tuberculosis (Edinb) 92, 211–217 (2012).
Lee, Y.K. et al. Late developmental plasticity in the T helper 17 lineage. Immunity 30, 92–107 (2009).
Martin-Orozco, N ., Chung, Y ., Chang, S.H ., Wang, Y.H . & Dong, C. Th17 cells promote pancreatic inflammation but only induce diabetes efficiently in lymphopenic hosts after conversion into Th1 cells. Eur. J. Immunol. 39, 216–224 (2009).
Nandi, B . & Behar, S.M. Regulation of neutrophils by interferon-{gamma} limits lung inflammation during tuberculosis infection. J. Exp. Med. 208, 2251–2262 (2011).
Wozniak, T.M ., Saunders, B.M ., Ryan, A.A . & Britton, W.J. Mycobacterium bovis BCG-specific Th17 cells confer partial protection against Mycobacterium tuberculosis infection in the absence of gamma interferon. Infect. Immun. 78, 4187–4194 (2010).
Vilaplana, C. et al. To achieve an earlier IFN-gamma response is not sufficient to control Mycobacterium tuberculosis infection in mice. PLoS One 9, e100830 (2014).
Rangel-Moreno, J. et al. The development of inducible bronchus-associated lymphoid tissue depends on IL-17. Nat. Immunol. 12, 639–646 (2011).
Torrado, E. et al. Differential and site specific impact of B cells in the protective immune response to Mycobacterium tuberculosis in the mouse. PLoS One 8, e61681 (2013).
Khader, S.A. et al. IL-23 is required for long-term control of Mycobacterium tuberculosis and B cell follicle formation in the infected lung. J. Immunol. 187, 5402–5407 (2011).
Cruz, A. et al. Pathological role of interleukin 17 in mice subjected to repeated BCG vaccination after infection with Mycobacterium tuberculosis. J. Exp. Med. 207, 1609–1616 (2010).
Ghilardi, N. et al. Compromised humoral and delayed-type hypersensitivity responses in IL-23-deficient mice. J. Immunol. 172, 2827–2833 (2004).
Acknowledgements
This work was supported by Children’s Hospital of Pittsburgh, Washington University in St Louis, NIH grant HL105427 to S.A.K., Children’s Hospital of Pittsburgh Research Advisory Committee Grant from Children’s Hospital of Pittsburgh of the UPMC Health System to L.M., American Lung Association Senior Research Training Fellowship RT-30592, Department of Molecular Microbiology, Washington University St Louis, and Alexander and Gertrude Berg Fellowship to K.L.G. J.R.-M. was supported by funds of the Department of Medicine, University of Rochester, and U19 AI91036.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declared no conflict of interest.
Additional information
SUPPLEMENTARY MATERIAL is linked to the online version of the paper
Supplementary information
Rights and permissions
About this article
Cite this article
Monin, L., Griffiths, K., Slight, S. et al. Immune requirements for protective Th17 recall responses to Mycobacterium tuberculosis challenge. Mucosal Immunol 8, 1099–1109 (2015). https://doi.org/10.1038/mi.2014.136
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/mi.2014.136
This article is cited by
-
Identification of perturbed pathways rendering susceptibility to tuberculosis in type 2 diabetes mellitus patients using BioNSi simulation of integrated networks of implicated human genes
Journal of Biosciences (2022)
-
A Mycobacterium tuberculosis-specific subunit vaccine that provides synergistic immunity upon co-administration with Bacillus Calmette-Guérin
Nature Communications (2021)
-
Increased Th17 activation and gut microbiota diversity are associated with pembrolizumab-triggered tuberculosis
Cancer Immunology, Immunotherapy (2020)
-
Mucosal boosting of H56:CAF01 immunization promotes lung-localized T cells and an accelerated pulmonary response to Mycobacterium tuberculosis infection without enhancing vaccine protection
Mucosal Immunology (2019)
-
Circulating Mycobacterium tuberculosis DosR latency antigen-specific, polyfunctional, regulatory IL10+ Th17 CD4 T-cells differentiate latent from active tuberculosis
Scientific Reports (2017)