Abstract
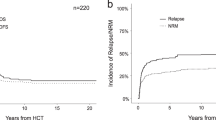
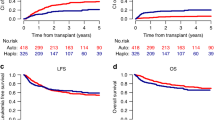
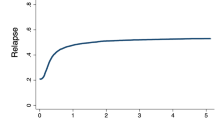
We evaluated the impact of salvage regimens and allogeneic hematopoietic cell transplantation (allo-HCT) in acute myeloid leukemia (AML) with induction failure. Between 1993 and 2009, 3324 patients with newly diagnosed AML were enrolled in 5 prospective treatment trials of the German-Austrian AML Study Group. After first induction therapy with idarubicin, cytarabine and etoposide (ICE), 845 patients had refractory disease. In addition, 180 patients, although responding to first induction, relapsed after second induction therapy. Of the 1025 patients with induction failure, 875 (median age 55 years) received intensive salvage therapy: 7+3-based (n=59), high-dose cytarabine combined with mitoxantrone (HAM; n=150), with all-trans retinoic acid (A; A-HAM) (n=247), with gemtuzumab ozogamicin and A (GO; GO-A-HAM) (n=140), other intensive regimens (n=165), experimental treatment (n=27) and direct allo-HCT (n=87). In patients receiving intensive salvage chemotherapy (n=761), response (complete remission/complete remission with incomplete hematological recovery (CR/CRi)) was associated with GO-A-HAM treatment (odds ratio (OR), 1.93; P=0.002), high-risk cytogenetics (OR, 0.62; P=0.006) and age (OR for a 10-year difference, 0.75; P<0.0001). Better survival probabilities were seen in an extended Cox regression model with time-dependent covariables in patients responding to salvage therapy (P<0.0001) and having the possibility to perform an allo-HCT (P<0.0001). FLT3 internal tandem duplication, mutated IDH1 and adverse cytogenetics were unfavorable factors for survival.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
References
Schlenk RF, Benner A, Hartmann F, del Valle F, Weber C, Pralle H et al. Risk-adapted postremission therapy in acute myeloid leukemia: results of the German multicenter AML HD93 treatment trial. Leukemia 2003; 17: 1521–1528.
Kern W, Haferlach T, Schoch C, Löffler H, Gassmann W, Heinecke A et al. Early blast clearance by remission induction therapy is a major independent prognostic factor for both achievement of complete remission and long-term outcome in acute myeloid leukemia: data from the German AML Cooperative Group (AMLCG) 1992 Trial. Blood 2003; 101: 64–70.
Wheatley K, Burnett AK, Goldstone AH, Gray RG, Hann IM, Harrison CJ et al. A simple, robust, validated and highly predictive index for the determination of risk-directed therapy in acute myeloid leukaemia derived from the MRC AML 10 trial. Br J Haematol 1999; 107: 69–79.
Döhner H, Estey EH, Amadori S, Appelbaum FR, Büchner T, Burnett AK et al. Diagnosis and management of acute myeloid leukemia in adults: recommendations from an international expert panel, on behalf of the European LeukemiaNet. Blood 2010; 115: 453–474.
Thol F, Schlenk RF, Heuser M, Ganser A . How I treat refractory and early relapsed acute myeloid leukemia. Blood 126: 319–327.
Ferguson P, Hills RK, Grech A, Betteridge S, Kjeldsen L, Dennis M et al. An operational definition of primary refractory acute myeloid leukemia allowing early identification of patients who may benefit from allogeneic stem cell transplantation. Haematologica 2016; 101: 1351–1358.
Ravandi F . Primary refractory acute myeloid leukaemia - in search of better definitions and therapies. Br J Haematol 2011; 155: 413–419.
Ravandi F, Cortes J, Faderl S, O'Brien S, Garcia-Manero G, Verstovsek S et al. Characteristics and outcome of patients with acute myeloid leukemia refractory to 1 cycle of high-dose cytarabine-based induction chemotherapy. Blood 2010; 116: 5818–5823.
Willemze R, Suciu S, Muus P, Halkes CJ, Meloni G, Meert L et al. Clofarabine in combination with a standard remission induction regimen (cytosine arabinoside and idarubicin) in patients with previously untreated intermediate and bad-risk acute myelogenous leukemia (AML) or high-risk myelodysplastic syndrome (HR-MDS): phase I results of an ongoing phase I/II study of the leukemia groups of EORTC and GIMEMA (EORTC GIMEMA 06061/AML-14A trial). Ann Hematol 2014; 93: 965–975.
Milligan DW, Wheatley K, Littlewood T, Craig, Jenny I O, Burnett AK . Fludarabine and cytosine are less effective than standard ADE chemotherapy in high-risk acute myeloid leukemia, and addition of G-CSF and ATRA are not beneficial: results of the MRC AML-HR randomized trial. Blood 2006; 107: 4614–4622.
Löwenberg B, Ossenkoppele GJ, van Putten W, Schouten HC, Graux C, Ferrant A et al. High-dose daunorubicin in older patients with acute myeloid leukemia. N Engl J Med 2009; 361: 1235–1248.
Fernandez HF, Sun Z, Yao X, Litzow MR, Luger SM, Paietta EM et al. Anthracycline dose intensification in acute myeloid leukemia. N Engl J Med 2009; 361: 1249–1259.
Lee JH, Joo YD, Kim H, Bae SH, Kim MK, Zang DY et al. A randomized trial comparing standard versus high-dose daunorubicin induction in patients with acute myeloid leukemia. Blood 2011; 118: 3832–3841.
Burnett AK, Russell NH, Hills RK, Kell J, Cavenagh J, Kjeldsen L et al. A randomized comparison of daunorubicin 90 mg/m2 vs 60 mg/m2 in AML induction: results from the UK NCRI AML17 trial in 1206 patients. Blood 2015; 125: 3878–3885.
Trifilio S, Zhou Z, Mehta J, Czerniak C, Pi J, Greenberg D, Koslosky M et al. Idarubicin appears equivalent to dose-intense daunorubicin for remission induction in patients with acute myeloid leukemia. Leuk Res 2013; 37: 868–871.
Pautas C, Merabet F, Thomas X, Raffoux E, Gardin C, Corm S et al. Randomized study of intensified anthracycline doses for induction and recombinant interleukin-2 for maintenance in patients with acute myeloid leukemia age 50 to 70 years: results of the ALFA-9801 study. J Clin Oncol 2010; 28: 808–814.
Gardin C, Chevret S, Pautas C, Turlure P, Raffoux E, Thomas X et al. Superior long-term outcome with idarubicin compared with high-dose daunorubicin in patients with acute myeloid leukemia age 50 years and older. J Clin Oncol 2013; 31: 321–327.
Kern W, Estey EH . High-dose cytosine arabinoside in the treatment of acute myeloid leukemia: Review of three randomized trials. Cancer 2006; 107: 116–124.
Willemze R, Suciu S, Meloni G, Labar B, Marie JP, Halkes CJ et al. High-dose cytarabine in induction treatment improves the outcome of adult patients younger than age 46 years with acute myeloid leukemia: results of the EORTC-GIMEMA AML-12 trial. J Clin Oncol 2014; 32: 219–228.
Schlenk RF, Fröhling S, Hartmann F, Fischer JT, Glasmacher A, del Valle F et al. Phase III study of all-trans retinoic acid in previously untreated patients 61 years or older with acute myeloid leukemia. Leukemia 2004; 18: 1798–1803.
Burnett AK, Hills RK, Green C, Jenkinson S, Koo K, Patel Y et al. The impact on outcome of the addition of all-trans retinoic acid to intensive chemotherapy in younger patients with nonacute promyelocytic acute myeloid leukemia: overall results and results in genotypic subgroups defined by mutations in NPM1, FLT3, and CEBPA. Blood 2010; 115: 948–956.
Thol F, Schlenk RF . Gemtuzumab ozogamicin in acute myeloid leukemia revisited. Expert Opin Biol Ther 2014; 14: 1185–1195.
Hills RK, Castaigne S, Appelbaum FR, Delaunay J, Petersdorf S, Othus M et al. Addition of gemtuzumab ozogamicin to induction chemotherapy in adult patients with acute myeloid leukaemia: a meta-analysis of individual patient data from randomised controlled trials. Lancet Oncol 2014; 15: 986–996.
Büchner T, Schlenk RF, Schaich M, Döhner K, Krahl R, Krauter J et al. Acute myeloid leukemia (AML): different treatment strategies versus a common standard arm—combined prospective analysis by the German AML Intergroup. J Clin Oncol 2012; 30: 3604–3610.
Juliusson G, Antunovic P, Derolf A, Lehmann S, Möllgård L, Stockelberg D et al. Age and acute myeloid leukemia: real world data on decision to treat and outcomes from the Swedish Acute Leukemia Registry. Blood 2009; 113: 4179–4187.
Schlenk RF, Döhner H . Genomic applications in the clinic: use in treatment paradigm of AML. Am Soc Hematol Educ Program 2013; 2013: 324–330.
Craddock C, Labopin M, Pillai S, Finke J, Bunjes D, Greinix H et al. Factors predicting outcome after unrelated donor stem cell transplantation in primary refractory acute myeloid leukaemia. Leukemia 2011; 25: 808–813.
Schmid C, Schleuning M, Schwerdtfeger R, Hertenstein B, Mischak-Weissinger E, Bunjes D et al. Long-term survival in refractory acute myeloid leukemia after sequential treatment with chemotherapy and reduced-intensity conditioning for allogeneic stem cell transplantation. Blood 2006; 108: 1092–1099.
Schlenk RF, Dohner K, Mack S, Stoppel M, Király F, Götze K et al. Prospective evaluation of allogeneic hematopoietic stem-cell transplantation from matched related and matched unrelated donors in younger adults with high-risk acute myeloid leukemia: German-Austrian trial AMLHD98A. J Clin Oncol 2010; 28: 4642–4648.
Montillo M, Mirto S, Petti MC, Latagliata R, Magrin S, Pinto A et al. Fludarabine, cytarabine, and G-CSF (FLAG) for the treatment of poor risk acute myeloid leukemia. Am J Hematol 1998; 58: 105–109.
Jackson G, Taylor P, Smith GM, Marcus R, Smith A, Chu P et al. A multicentre, open, non-comparative phase II study of a combination of fludarabine phosphate, cytarabine and granulocyte colony-stimulating factor in relapsed and refractory acute myeloid leukaemia and de novo refractory anaemia with excess of blasts in transformation. Br J Haematol 2001; 112: 127–137.
Camera A, Rinaldi CR, Palmieri S, Cantore N, Mele G, Mettivier V et al. Sequential continuous infusion of fludarabine and cytarabine associated with liposomal daunorubicin (DaunoXome) (FLAD) in primary refractory or relapsed adult acute myeloid leukemia patients. Ann Hematol 2009; 88: 151–158.
Fiegl M, Unterhalt M, Kern W, Braess J, Spiekermann K, Staib P et al. Chemomodulation of sequential high-dose cytarabine by fludarabine in relapsed or refractory acute myeloid leukemia: a randomized trial of the AMLCG. Leukemia 2014; 28: 1001–1007.
Faderl S, Wetzler M, Rizzieri D, Schiller G, Jagasia M, Stuart R et al. Clofarabine plus cytarabine compared with cytarabine alone in older patients with relapsed or refractory acute myelogenous leukemia: results from the CLASSIC I Trial. J Clin Oncol 2012; 30: 2492–2499.
Ravandi F, Ritchie EK, Sayar H, Lancet JE, Craig MD, Vey N et al. Vosaroxin plus cytarabine versus placebo plus cytarabine in patients with first relapsed or refractory acute myeloid leukaemia (VALOR): a randomised, controlled, double-blind, multinational, phase 3 study. Lancet Oncol 2015; 16: 1025–1036.
Rein LA, Rizzieri DA . Clinical potential of elacytarabine in patients with acute myeloid leukemia. Ther Adv Hematol 2014; 5: 211–220.
Roboz GJ, Rosenblat T, Arellano M, Gobbi M, Altman JK, Montesinos P et al. International randomized phase III study of elacytarabine versus investigator choice in patients with relapsed/refractory acute myeloid leukemia. J Clin Oncol 2014; 32: 1919–1926.
Fröhling S, Schlenk RF, Kayser S, Morhardt M, Benner A, Döhner K et al. Cytogenetics and age are major determinants of outcome in intensively treated acute myeloid leukemia patients older than 60 years: results from AMLSG trial AML HD98-B. Blood 2006; 108: 3280–3288.
Tassara M, Dohner K, Brossart P, Held G, Götze K, Horst HA et al. Valproic acid in combination with all-trans retinoic acid and intensive therapy for acute myeloid leukemia in older patients. Blood 2014; 123: 4027–4036.
Schlenk RF, Lübbert M, Benner A, Lamparter A, Krauter J, Herr W et al. All-trans retinoic acid as adjunct to intensive treatment in younger adult patients with acute myeloid leukemia: results of the randomized AMLSG 07-04 study. Ann Hematol 2016; 95: 1931–1942.
Bennett JM, Catovsky D, Daniel MT, Flandrin G, Galton DA, Gralnick HR et al. Proposals for the classification of the acute leukaemias. French-American-British (FAB) co-operative group. Br J Haematol 1976; 33: 451–458.
Vardiman JW, Harris NL, Brunning RD . The World Health Organization (WHO) classification of the myeloid neoplasms. Blood 2002; 100: 2292–2302.
Hütter-Krönke ML, Benner A, Döhner K, Krauter J, Weber D, Moessner M et al. Salvage therapy with high-dose cytarabine and mitoxantrone in combination with all-trans retinoic acid and gemtuzumab ozogamicin in acute myeloid leukemia refractory to first induction therapy. Haematologica 2016; 101: 839–845.
Burnett AK, Russell NH, Hills RK, Hunter AE, Kjeldsen L, Yin J et al. Optimization of chemotherapy for younger patients with acute myeloid leukemia: results of the medical research council AML15 trial. J Clin Oncol 2013; 31: 3360–3368.
Luo S, Cai F, Jiang L, Zhang S, Shen Z, Sun L et al. Clinical study of Mito-FLAG regimen in treatment of relapsed acute myeloid leukemia. Exp Ther Med 2013; 5: 982–986.
Mitelman F (ed) ISCN (1995): An International System for Human Cytogenetic Nomenclature. Karger: Basel, Switzerland, 1995.
Schlenk RF, Döhner K, Krauter J, Fröhling S, Corbacioglu A, Bullinger L et al. Mutations and treatment outcome in cytogenetically normal acute myeloid leukemia. N Engl J Med 2008; 358: 1909–1918.
Paschka P, Schlenk RF, Gaidzik VI, Herzig JK, Aulitzky T, Bullinger L et al. ASXL1 mutations in younger adult patients with acute myeloid leukemia: a study by the German-Austrian Acute Myeloid Leukemia Study Group. Haematologica 2015; 100: 324–330.
Schemper M, Smith TL . A note on quantifying follow-up in studies of failure time. Control Clin Trials 1996; 17: 343–346.
Andersen P, Gill RD . Cox’s regression model for counting processes: a large sample study. Ann Stat 1982; 10: 1100–1120.
Harrell FE . Regression Modeling Strategies: With Applications to Linear Models, Logistic Regression, and Survival Analysis. Springer: New York, NY, 2001.
R Development Core Team R: A Language and Environment for Statistical Computing. R Foundation for Statistical Computing: Vienna, Austria, 2009.
Schlenk RF, Kayser S, Bullinger L, Kobbe G, Casper J, Ringhoffer M et al. Differential impact of allelic ratio and insertion site in FLT3-ITD-positive AML with respect to allogeneic transplantation. Blood 2014; 124: 3441–3449.
Pfeiffer T, Schleuning M, Mayer J, Haude KH, Tischer J, Buchholz S et al. Influence of molecular subgroups on outcome of acute myeloid leukemia with normal karyotype in 141 patients undergoing salvage allogeneic stem cell transplantation in primary induction failure or beyond first relapse. Haematologica 2013; 98: 518–525.
Acknowledgements
This work was supported by grants from German Bundesministerium für Bildung und Forschung (Grants 01GI9981,01KG0605) and the Deutsche José Carreras Leukämie-Stiftung (Grant DJCLS H 05/02). We are also grateful to all members of the German-Austrian AML Study Group (AMLSG) for providing leukemia specimens and clinical data; a list of AMLSG institutions and investigators participating in this study appears in the Supplementary Appendix.
Author contributions
Conception and design: RF Schlenk and M Wattad; provision of study materials or patients: M Wattad, D Weber, K Döhner, J Krauter, VI Gaidzik, P Paschka, M Heuser, F Thol, T Kindler, M Lübbert, HR Salih, A Kündgen, H-A Horst, P Brossart, K Götze, D Nachbaur, C-H Köhne, M Ringhoffer, G Wulf, G Held, H Salwender, A Benner, A Ganser, H Döhner, and RF Schlenk; collection and assembly of data: RF Schlenk and D Weber; data analysis and interpretation: RF Schlenk, M Wattad, H Döhner and A Benner; manuscript writing: M Wattad, RF Schlenk and H Döhner; final approval of manuscript: M Wattad, D Weber, K Döhner, J Krauter, VI Gaidzik, P Paschka, M Heuser, Fs Thol, T Kindler, M Lübbert, HR Salih, A Kündgen, H-A Horst, P Brossart, K Götze, D Nachbaur, C-H Köhne, M Ringhoffer, G Wulf, G Held, H Salwender, A Benner, A Ganser, H Döhner and RF Schlenk.
Author information
Authors and Affiliations
Consortia
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Additional information
Presented in part at the 19th Congress of the European Hematology Association, 2014.
Supplementary Information accompanies this paper on the Leukemia website
Supplementary information
Rights and permissions
About this article
Cite this article
Wattad, M., Weber, D., Döhner, K. et al. Impact of salvage regimens on response and overall survival in acute myeloid leukemia with induction failure. Leukemia 31, 1306–1313 (2017). https://doi.org/10.1038/leu.2017.23
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/leu.2017.23
This article is cited by
-
Allogeneic hematopoietic cell transplantation for older patients with AML with active disease. A study from the Acute Leukemia Working Party of the European Society for Blood and Marrow Transplantation (EBMT)
Bone Marrow Transplantation (2024)
-
Re-induction therapy in patients with acute myeloid leukemia not in complete remission after the first course of treatment
Annals of Hematology (2023)
-
Therapie der rezidivierten/refraktären akuten myeloischen Leukämie
Im Fokus Onkologie (2023)
-
Q-HAM: a multicenter upfront randomized phase II trial of quizartinib and high-dose Ara-C plus mitoxantrone in relapsed/refractory AML with FLT3-ITD
Trials (2023)
-
European LeukemiaNet-defined primary refractory acute myeloid leukemia: the value of allogeneic hematopoietic stem cell transplant and overall response
Blood Cancer Journal (2022)