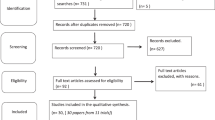
Abstract
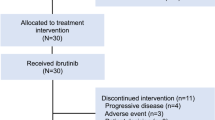
We examined the combination of the mammalian target of rapamycin inhibitor everolimus with bortezomib and rituximab in patients with relapsed/refractory Waldenstrom macroglobulinemia (WM) in a phase I/II study. All patients received six cycles of the combination of everolimus/rituximab or everolimus/bortezomib/rituximab followed by maintenance with everolimus until progression. Forty-six patients were treated; 98% received prior rituximab and 57% received prior bortezomib. No dose-limiting toxicities were observed in the phase I. The most common treatment-related toxicities of all grades were fatigue (63%), anemia (54%), leucopenia (52%), neutropenia (48%) and diarrhea (43%). Thirty-six (78%) of the 46 patients received full dose therapy (FDT) of the three drugs. Of these 36, 2 (6%) had complete response (90% confidence interval (CI): 1–16). In all, 32/36 (89%) of patients experienced at least a minimal response (90% CI: 76–96%). The observed partial response or better response rate was 19/36 (53, 90 CI: 38–67%). For the 36 FDT patients, the median progression-free survival was 21 months (95% CI: 12–not estimable). In summary, this study demonstrates that the combination of everolimus, bortezomib and rituximab is well tolerated and achieved 89% response rate even in patients previously treated, making it a possible model of non-chemotherapeutic-based combination therapy in WM.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Sahin I, Leblebjian H, Treon SP, Ghobrial IM . Waldenstrom macroglobulinemia: from biology to treatment. Expert Rev Hematol 2014; 7: 157–168.
Treon SP, Hunter ZR, Castillo JJ, Merlini G . Waldenstrom macroglobulinemia. Hematol Oncol Clin North Am 2014; 28: 945–970.
Dimopoulos MA, Panayiotidis P, Moulopoulos LA, Sfikakis P, Dalakas M . Waldenstrom's macroglobulinemia: clinical features, complications, and management. J Clin Oncol 2000; 18: 214–226.
Ghobrial IM, Witzig TE . Waldenstrom macroglobulinemia. Curr Treat Options Oncol 2004; 5: 239–247.
Dimopoulos MA, Kyle RA, Anagnostopoulos A, Treon SP . Diagnosis and management of Waldenstrom's macroglobulinemia. J Clin Oncol 2005; 23: 1564–1577.
Owen RG, Treon SP, Al-Katib A, Fonseca R, Greipp PR, McMaster ML et al. Clinicopathological definition of Waldenstrom's macroglobulinemia: consensus panel recommendations from the Second International Workshop on Waldenstrom's Macroglobulinemia. Semin Oncol 2003; 30: 110–115.
Leblond V, Johnson S, Chevret S, Copplestone A, Rule S, Tournilhac O et al. Results of a randomized trial of chlorambucil versus fludarabine for patients with untreated Waldenstrom macroglobulinemia, marginal zone lymphoma, or lymphoplasmacytic lymphoma. J Clin Oncol 2013; 31: 301–307.
Anderson KC, Alsina M, Bensinger W, Biermann JS, Cohen AD, Devine S et al. Waldenstrom's macroglobulinemia/lymphoplasmacytic lymphoma, version 2.2013. J Natl Compr Canc Netw 2012; 10: 1211–1219.
Ghobrial IM, Hong F, Padmanabhan S, Badros A, Rourke M, Leduc R et al. Phase II trial of weekly bortezomib in combination with rituximab in relapsed or relapsed and refractory Waldenstrom macroglobulinemia. J Clin Oncol 2010; 28: 1422–1428.
Ghobrial IM, Xie W, Padmanabhan S, Badros A, Rourke M, Leduc R et al. Phase II trial of weekly bortezomib in combination with rituximab in untreated patients with Waldenstrom macroglobulinemia. Am J Hematol 2010; 85: 670–674.
Dimopoulos MA, Chen C, Kastritis E, Gavriatopoulou M, Treon SP . Bortezomib as a treatment option in patients with Waldenstrom macroglobulinemia. Clin Lymphoma Myeloma Leuk 2010; 10: 110–117.
Treon SP, Ioakimidis L, Soumerai JD, Patterson CJ, Sheehy P, Nelson M et al. Primary therapy of Waldenstrom macroglobulinemia with bortezomib, dexamethasone, and rituximab: WMCTG clinical trial 05-180. J Clin Oncol 2009; 27: 3830–3835.
Treon SP, Xu L, Yang G, Zhou Y, Liu X, Cao Y et al. MYD88 L265P somatic mutation in Waldenstrom's macroglobulinemia. N Engl J Med 2012; 367: 826–833.
Xu L, Hunter ZR, Yang G, Zhou Y, Cao Y, Liu X et al. MYD88 L265P in Waldenstrom's macroglobulinemia, IgM monoclonal gammopathy, and other B-cell lymphoproliferative disorders using conventional and quantitative allele-specific PCR. Blood 2013; 121: 2051–2058.
Laird MH, Rhee SH, Perkins DJ, Medvedev AE, Piao W, Fenton MJ et al. TLR4/MyD88/PI3K interactions regulate TLR4 signaling. J Leukoc Biol 2009; 85: 966–977.
Troutman TD, Hu W, Fulenchek S, Yamazaki T, Kurosaki T, Bazan JF et al. Role for B-cell adapter for PI3K (BCAP) as a signaling adapter linking Toll-like receptors (TLRs) to serine/threonine kinases PI3K/Akt. Proc Natl Acad Sci USA 2012; 109: 273–278.
Roccaro AM, Sacco A, Jimenez C, Maiso P, Moschetta M, Mishima Y et al. C1013G/CXCR4 acts as a driver mutation of tumor progression and modulator of drug resistance in lymphoplasmacytic lymphoma. Blood 2014; 123: 4120–4131.
Hunter ZR, Xu L, Yang G, Zhou Y, Liu X, Cao Y et al. The genomic landscape of Waldenstrom macroglobulinemia is characterized by highly recurring MYD88 and WHIM-like CXCR4 mutations, and small somatic deletions associated with B-cell lymphomagenesis. Blood 2014; 123: 1637–1646.
Treon SP, Cao Y, Xu L, Yang G, Liu X, Hunter ZR . Somatic mutations in MYD88 and CXCR4 are determinants of clinical presentation and overall survival in Waldenstrom's macroglobulinemia. Blood 2014; 123: 2791–2796.
Zhang Y, Roccaro AM, Rombaoa C, Flores L, Obad S, Fernandes SM et al. LNA-mediated anti-miR-155 silencing in low-grade B-cell lymphomas. Blood 2012; 120: 1678–1686.
Roccaro AM, Sacco A, Chen C, Runnels J, Leleu X, Azab F et al. microRNA expression in the biology, prognosis, and therapy of Waldenstrom macroglobulinemia. Blood 2009; 113: 4391–4402.
Roccaro AM, Sacco A, Jia X, Banwait R, Maiso P, Azab F et al. Mechanisms of activity of the TORC1 inhibitor everolimus in Waldenstrom macroglobulinemia. Clin Cancer Res 2012; 18: 6609–6622.
Motzer RJ, Escudier B, Oudard S, Hutson TE, Porta C, Bracarda S et al. Efficacy of everolimus in advanced renal cell carcinoma: a double-blind, randomised, placebo-controlled phase III trial. Lancet 2008; 372: 449–456.
Treon SP . How I treat Waldenstrom macroglobulinemia. Blood 2009; 114: 2375–2385.
Chan S . Targeting the mammalian target of rapamycin (mTOR): a new approach to treating cancer. Br J Cancer 2004; 91: 1420–1424.
Pene F, Claessens YE, Muller O, Viguie F, Mayeux P, Dreyfus F et al. Role of the phosphatidylinositol 3-kinase/Akt and mTOR/P70S6-kinase pathways in the proliferation and apoptosis in multiple myeloma. Oncogene 2002; 21: 6587–6597.
Foster DA . Targeting mTOR-mediated survival signals in anticancer therapeutic strategies. Expert Rev Anticancer Ther 2004; 4: 691–701.
Shi Y, Sharma A, Wu H, Lichtenstein A, Gera J . Cyclin D1 and c-Myc internal ribosome entry site (IRES)-dependent translation is regulated by AKT activity and enhanced by rapamycin through a p38 MAPK and ERK-dependent pathway. J Biol Chem 2005; 280: 10964–10973.
Choo AY, Blenis J . TORgeting oncogene addiction for cancer therapy. Cancer Cell 2006; 9: 77–79.
Thoreen CC, Kang SA, Chang JW, Liu Q, Zhang J, Gao Y et al. An ATP-competitive mammalian target of rapamycin inhibitor reveals rapamycin-resistant functions of mTORC1. J Biol Chem 2009; 284: 8023–8032.
Ghobrial IM, Gertz M, Laplant B, Camoriano J, Hayman S, Lacy M et al. Phase II trial of the oral mammalian target of rapamycin inhibitor everolimus in relapsed or refractory Waldenstrom macroglobulinemia. J Clin Oncol 2010; 28: 1408–1414.
Ghobrial IM, Witzig TE, Gertz M, LaPlant B, Hayman S, Camoriano J et al. Long-term results of the phase II trial of the oral mTOR inhibitor everolimus (RAD001) in relapsed or refractory Waldenstrom Macroglobulinemia. Am J Hematol 2014; 89: 237–242.
Dimopoulos MA, Kastritis E, Owen RG, Kyle RA, Landgren O, Morra E et al. Treatment recommendations for patients with Waldenstrom macroglobulinemia (WM) and related disorders: IWWM-7 consensus. Blood 2014; 124: 1404–1411.
Owen RG, Kyle RA, Stone MJ, Rawstron AC, Leblond V, Merlini G et al. Response assessment in Waldenstrom macroglobulinaemia: update from the VIth International Workshop. Br J Haematol 2013; 160: 171–176.
Morel P, Duhamel A, Gobbi P, Dimopoulos MA, Dhodapkar MV, McCoy J et al. International prognostic scoring system for Waldenstrom's macroglobulinemia. Blood 2009; 113: 4163–4170.
Cao Y, Hunter ZR, Liu X, Xu L, Yang G, Chen J et al. CXCR4 WHIM-like frameshift and nonsense mutations promote ibrutinib resistance but do not supplant MYD88 -directed survival signalling in Waldenstrom macroglobulinaemia cells. Br J Haematol 2014; 168: 701–707.
Byrd JC, Furman RR, Coutre SE, Flinn IW, Burger JA, Blum KA et al. Targeting BTK with ibrutinib in relapsed chronic lymphocytic leukemia. N Engl J Med 2013; 369: 32–42.
Acknowledgements
Supported in part by 1R01FD003743, Novartis Inc., Millenium/Takeda Inc. and the International Waldenstrom Macroglobulinemia Foundation (IWMF).
Author Contributions
Irene M Ghobrial: initiated and wrote the clinical trial and was the principal investigator at DFCI. She conducted the clinical trial, analyzed the data and wrote the manuscript, Robert Redd: performed the statistical analysis; Philippe Armand: enrolled patients and reviewed the manuscript; Erica Boswell: entered and analyzed the data and reviewed the manuscript; Stacey Chuma: was the research nurse of the study; Antonio Sacco: performed the mutation studies for MYD88 and CXCR4, Kimberly Noonan: assessed patients during therapy and follow-up; Ranjit Banwait: entered and analyzed the data and reviewed the manuscript; Houry Leblebjian: pharmacist of the study and reviewed treatment and dose modifications of the study; Diane Warren: project manager of the clinical study; Megan MacNabb: assistant project manager, managed the regulatory components of the study; Aldo M Roccaro: performed the mutation studies for MYD88 and CXCR4; Daisy Huynh: performed the mutation studies for MYD88 and CXCR4; Adriana Perilla-Glen performed the mutation studies for MYD88 and CXCR4; Patrick Henrick: entered and analyzed the data and reviewed the manuscript; Jorge Castillo: cared for patients in the study and reviewed the manuscript; Paul G Richardson: input in the design and conduct of the study and reviewed the manuscript; Jeffrey Matous enrolled patients and reviewed the manuscript; Edie Weller: performed the statistical analysis; Steven P Treon: input in the design and conduct of the study, enrolled patients and reviewed the manuscript.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
Irene M Ghobrial: Consultant for Novartis, Millennium, Celgene and BMS. Philip Armand: received funding from BMS, Merck, Otsuka, Sigma Tau and Sequenta; consultant for Merck. Paul Richardson: Consultant for Novartis, Millennium and Celgene. The rest of the authors declare no conflict of interest.
Rights and permissions
About this article
Cite this article
Ghobrial, I., Redd, R., Armand, P. et al. Phase I/II trial of everolimus in combination with bortezomib and rituximab (RVR) in relapsed/refractory Waldenstrom macroglobulinemia. Leukemia 29, 2338–2346 (2015). https://doi.org/10.1038/leu.2015.164
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/leu.2015.164
This article is cited by
-
Bortezomib for the Treatment of Hematologic Malignancies: 15 Years Later
Drugs in R&D (2019)
-
Waldenström macroglobulinemia treatment algorithm 2018
Blood Cancer Journal (2018)
-
Pharmacokinetically-targeted dosed everolimus maintenance therapy in lymphoma patients
Cancer Chemotherapy and Pharmacology (2018)